5.1.2 Biocontrol Agents Associated With Potato And Vegetable Pests![]() / Apanteles subandinus (Blanchard 1947)
/ Apanteles subandinus (Blanchard 1947)
Taxonomic position: Hymenoptera, Braconidae (Microgastrinae)
Authors: V. Cañedo, P. Carhuapoma, W. Dávila, & J. Kroschel
Hosts
Potato tuber moth, Phthorimaea operculella (Zeller); Andean potato tuber moth, Symmetrischema tangolias (Gyen); Guatemalan potato tuber moth, Tecia solanivora (Povolny)
Morphology
Egg
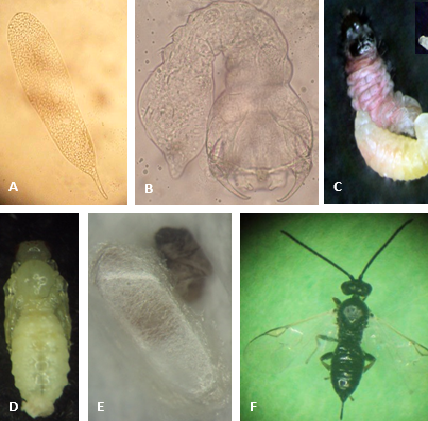
Elongated and translucid with a small pedicel about 0.50 x 0.08 mm in size (Photo 1A).
Larva
It has three instar larvae. The first instar is mandibulate-caudate type and measures approximately 0.41 x 0.18 mm (Photo 1B). The third instar is hymenopteriform and measures 3.06 x 0.73 mm (Photo 1C).
Pupa
The pupa is exarate type (appendages are free and not glued to the body) about 3.6 x 1.7 mm in size (Photo 1D). It develops inside the cocoon made by the mature last instar larva, soon after it emerges from the host (Photo 1E).
Adult
Males and females are black with palps and tibiae less dark. Females measure approximately 3.86 mm from the tip of the head to the tip of the abdomen (Photo 1F). The average length of the ovipositor is 0.64 mm. The male is somewhat smaller, averaging 3.64 mm in length. Both are very similar to males and females of A. scutellaris.
Biology
Parasitism
Apanteles subandinus is a solitary endoparasitoid of larvae, depositing eggs preferably into early instar larvae (L1-L2). The host’s parasitized larva generally develops and survives up to the last instar larvae, at which stage A. subandinus has fully completed its development. The female wasp searches with her antennae for a suitable host and is able to locate host food plants and discriminate between the volatiles of mechanically damaged plants and those infested with host larvae. They are also able to distinguish between infested and non-infested plants from a distance of 30 cm. The combination of odors originating from mechanically injured and P. operculella-damaged plants plays a crucial role in the foraging behavior of A. subandinus. Female wasps that developed in one particular host have more capacity to search for this specific host then for an alternative host. Females also increase their capacity to find an alternative host when they successfully parasitized this new host species. A. subandinus individuals became smaller when kept in continuous laboratory culture.
Temperature-dependent development
subandinus completes its development from egg to adult at temperatures of 15°–30°C but not at low temperatures of 11°C (see Annex 7.4.2). Total development at 15°C was almost 3.5 times longer (70.6 days) than at 30°C (18.4 days). The highest mortality of egg-larvae and pupa was 58% at 30°C and the lowest mortality of both stages, egg-larvae and pupa, was 33% and 16% at 25°C. Female longevity decreased with temperatures from 37 days at 15°C to 4 days at 30°C. The maximum fecundity of 145 offspring was observed at a temperature range of 26°–30°C. The sex ratio is highly affected by temperature, with a predominance of males at lower temperature of 15°C (1:6.6 for female:male). In the temperature range of 20°–30°C the sex ratio was almost 1:1.
The established functions were used to estimate the life-table parameters and to build an overall stochastic phenology model. Negative r values, under 13°C and above 32°C, indicate that population size is decreasing at these temperatures; positive r values of 15°–30°C indicate that the population is increasing. The finite rate of increase peaked at 28°C (λ=1.101) and was smallest at 35°C (λ=0.886) and 11°C (λ=0.973); λ<1 indicates that the population is decreasing. Highest values for the gross reproduction rate and net reproduction rate (Ro) were found between 28°C (26.48 offspring) and 25°C (8.71 offspring), respectively. The shortest mean generation time (T) was observed at 30°C (17.56 days); the shortest population doubling time (Dt) at 28°C (7.23 days). The optimum temperature for overall population growth ranged from 25°C to 28°C (see Annex 7.4.2).
Economic impact in pest control
In classical biological control programs of several countries, A. subandinus was released as an exotic parasitoid for controlling P. operculella (see section 4.1.1) often in combination with the encyrtid Copidosoma koehleri (Blanchard) and the braconid Orgilus lepidus (Muesebeck) (see sections 5.1.1 and 5.1.3). The parasitoid established successfully in several countries, but the control efficacy was not consistent among the regions. Most successful control was achieved in Zambia and Cyprus. In Zambia and Zimbabwe, A. subandinus was released together with C. koehleri (both imported from South Africa) in the 1960s. After the establishment of both parasitoids, after May 1969, the pest P. operculella became very scarce. In Zambia both parasitoids afforded almost complete control. In Australia, A. subandinus (first released in December 1964) occurred from Tasmania to Queensland, with >70% parasitism in some districts of Victoria. In April 1970, parasitism averaged 56.6% in 12 potato-growing districts. A. subandinus was reported as the most effective parasitoid in the northern areas of Queensland. More recent studies showed that in Victoria, A. subandinus and O. lepidus were the most abundant parasitoids.
Geographical distribution
Possible regions of origin: Andean region of South America (Peru) and Argentina. In Peru, A. subandinus was first reported in the 1960s, but its current presence could not be verified through new collections in many potato-growing areas.
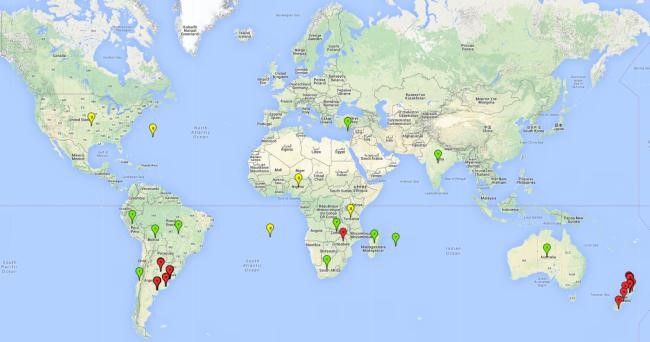
Introduced and established: Australia, Bolivia, Brazil, Chile, Cyprus, India, Madagascar, Mauritius, New Zealand (including Tasmania), South Africa, Uruguay, Zambia, and Zimbabwe.
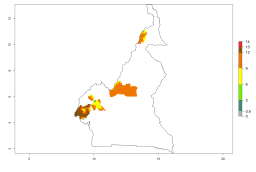
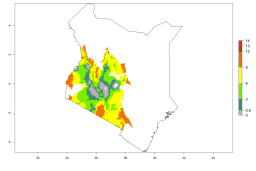
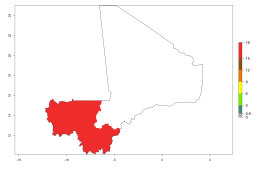
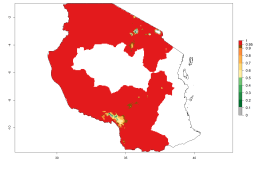
Introduced but establishment not confirmed: Bermuda, Nigeria, St. Helena, Tanzania, and USA (Fig. 1).
Potential establishment and efficiency under current and future climates
Changes in global establishment and future distribution
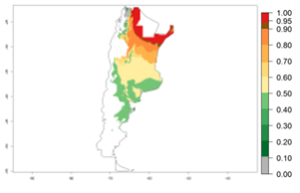
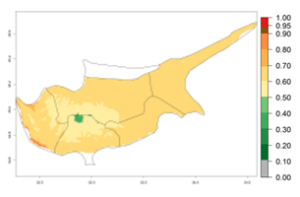
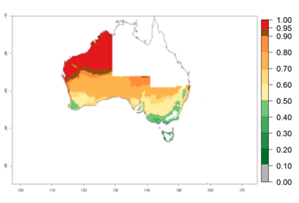
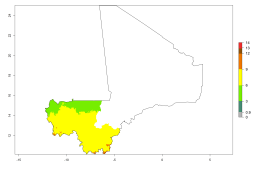
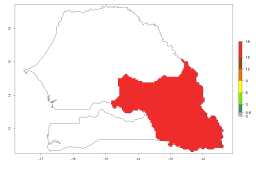
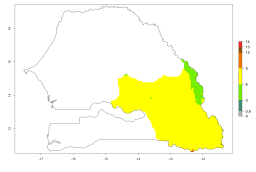
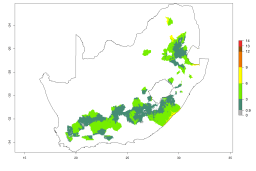
In potato production regions globally, the potato tuber moth, P. operculella, is potentially established at an establishment risk index (ERI)>0.7 (see section 4.1.1). Potential release regions for A. subandinus are those potato production regions where the potato tuber moth has been established, causing significant economic damage in potato fields and stores. Therefore, the establishment index (EI) for the parasitoid A. subandinus is only displayed for those regions where P. operculella can potentially establish. An EI=1 indicates survival of the parasitoid throughout the year—that is, the likelihood of long-term establishment for classical biological control is very high in these regions. However, A. subandinus has also been established in regions with an EI>0.5–0.9 (light green to orange zones) as in Argentina, Cyprus, and Australia (Fig. 2, compare with Fig. 1).
| Argentina | Cyprus | Australia |
Figure 2. EI of Apanteles subandinus in countries and regions where establishment has been reported according to model predictions of the year 2000. An EI>0.5 (light yellow to red zones) indicates regions with permanent establishment.
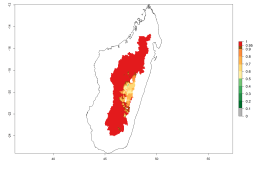
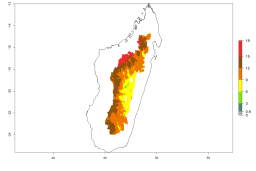
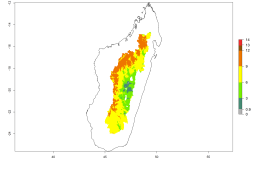
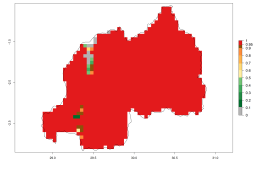
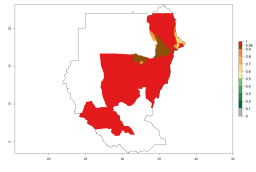
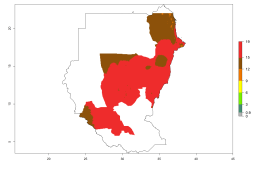
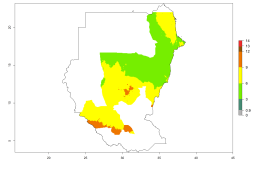
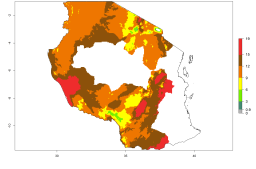
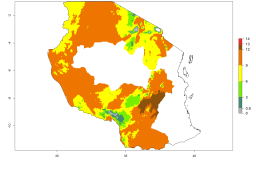
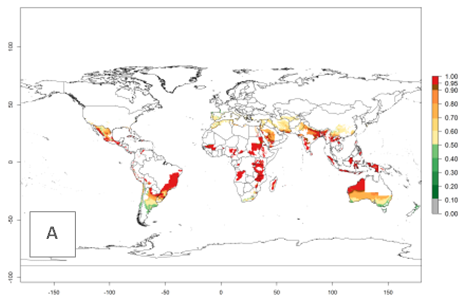
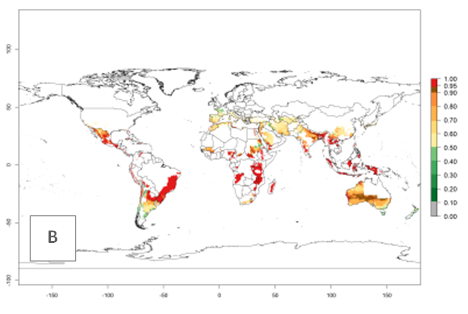
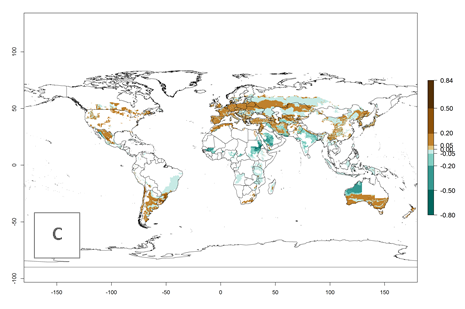
Many regions can be identified where A. subandinus has an EI>0.90. These are located in South America (Venezuela, Colombia, Ecuador, Peru, Bolivia, north of Argentina, southeast of Brazil); Central America (Mexico); in potato-growing regions of countries in Central and East Africa and Madagascar; Asia (India, Nepal, Indonesia); and northwest Australia (Fig. 3A). Global predictions for 2050 indicate that the establishment of A. subandinus will potentially increase (EI>0.95) in some potato-growing areas of South America (Peru, Bolivia, Chile, Argentina, Uruguay, Brazil); Mexico in Central America; and Africa (South Africa). A slight range expansion to more temperate, regions of southern South America (Fig. 3B) is projected. In contrast, the potential establishment decreases in some potato-growing areas of Africa, India, and regions from northwest of Australia (Fig. 3C).
 |
 |
 |
|
Figure 3. Changes in establishment and potential distribution worldwide of Apanteles subandinus, parasitoid of Phthorimaea operculella, according to model predictions, using the EI for the years 2000 (A) and 2050 (B), and changes of the EI between 2000 and 2050 (C) displayed in potato production regions globally with an ERI>0.7 of its primary host Phthorimaea operculella. An EI>0.5 indicates regions with potential permanent establishment
Changes in global abundance
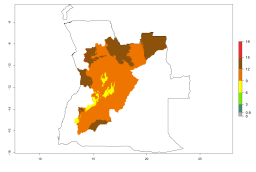
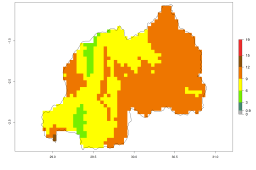
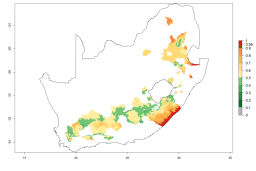
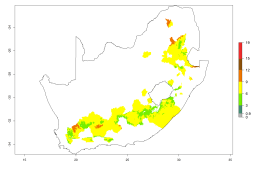
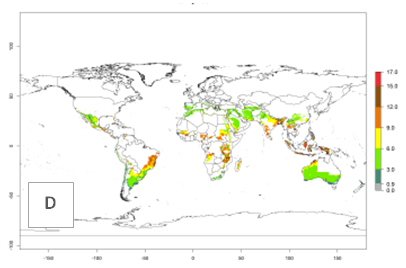
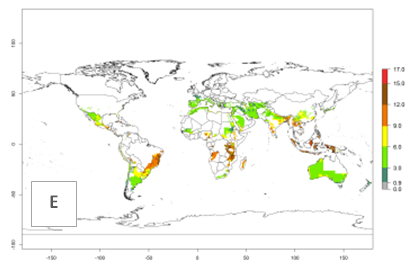
The generation index (GI) reflects the potential abundance of the parasitoid population, and it estimates the mean number of generations that A. subandinus may produce in potato production regions according to existing and predicted temperature conditions of the years 2000 and 2050 (Fig. 4A, B). The number of generations in countries in which A. subandinus has been established ranges 6–9 per year. For the year 2050, in some tropical areas of America (Mexico, Peru, Bolivia, Chile, southeast Brazil); Southern and East Africa; some Mediterranean countries; and Southeast Asia (Indonesia, some regions of India), the generation change index indicates a potential increase of 2–4 generations per year (Fig. 4C).
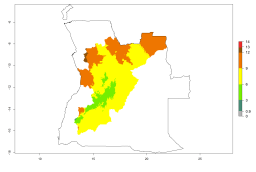
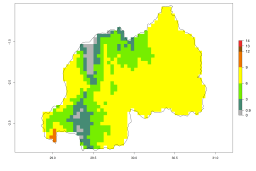
The activity index (AI) highlights not only the establishment but also the potential spread of an insect and, in the case of parasitoids, its potential to efficiently control its host. For the year 2000, an AI>3–9 is indicated for those countries where A. subandinus is present today (Fig. 4D, compare with Fig. 1). Predictions of change for the 2050 climate change scenario show an increase in the potential growth by a factor of 1–5 in some potato-growing areas of South America (Colombia, Peru, Bolivia, Chile, southeast Brazil); in some countries of southeast Africa; and in some regions of South Asia and south of Australia. In contrast, a reduction of the potential growth can be expected for regions of India, Indonesia, and Australia (Fig. 4E, F).
| GI | AI | |
| 2000 | 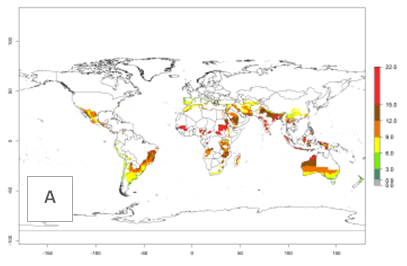 |
 |
| 2050 | 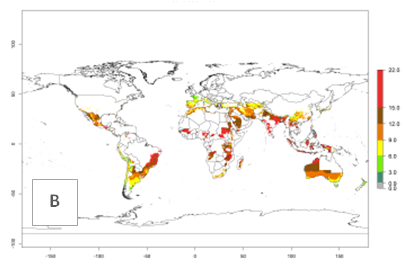 |
 |
| Index change (2000 – 2050) | 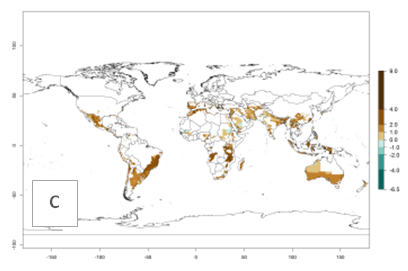 |
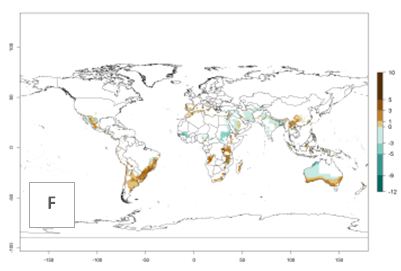 |
Figure 4. Changes in abundance (GI, number of generations/year) and activity (AI, potential population growth) of Apanteles subandinus in potato production regions worldwide according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F), displayed in potato production regions with an ERI>0.7 of its primary host Phthorimaea operculella.
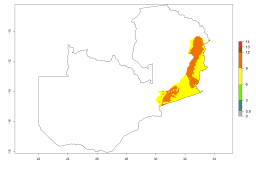
Changes in regional establishment and distribution in Africa
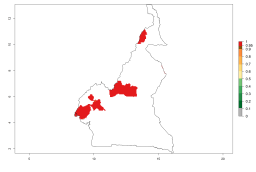
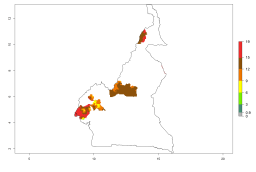
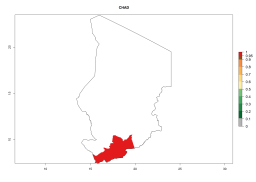
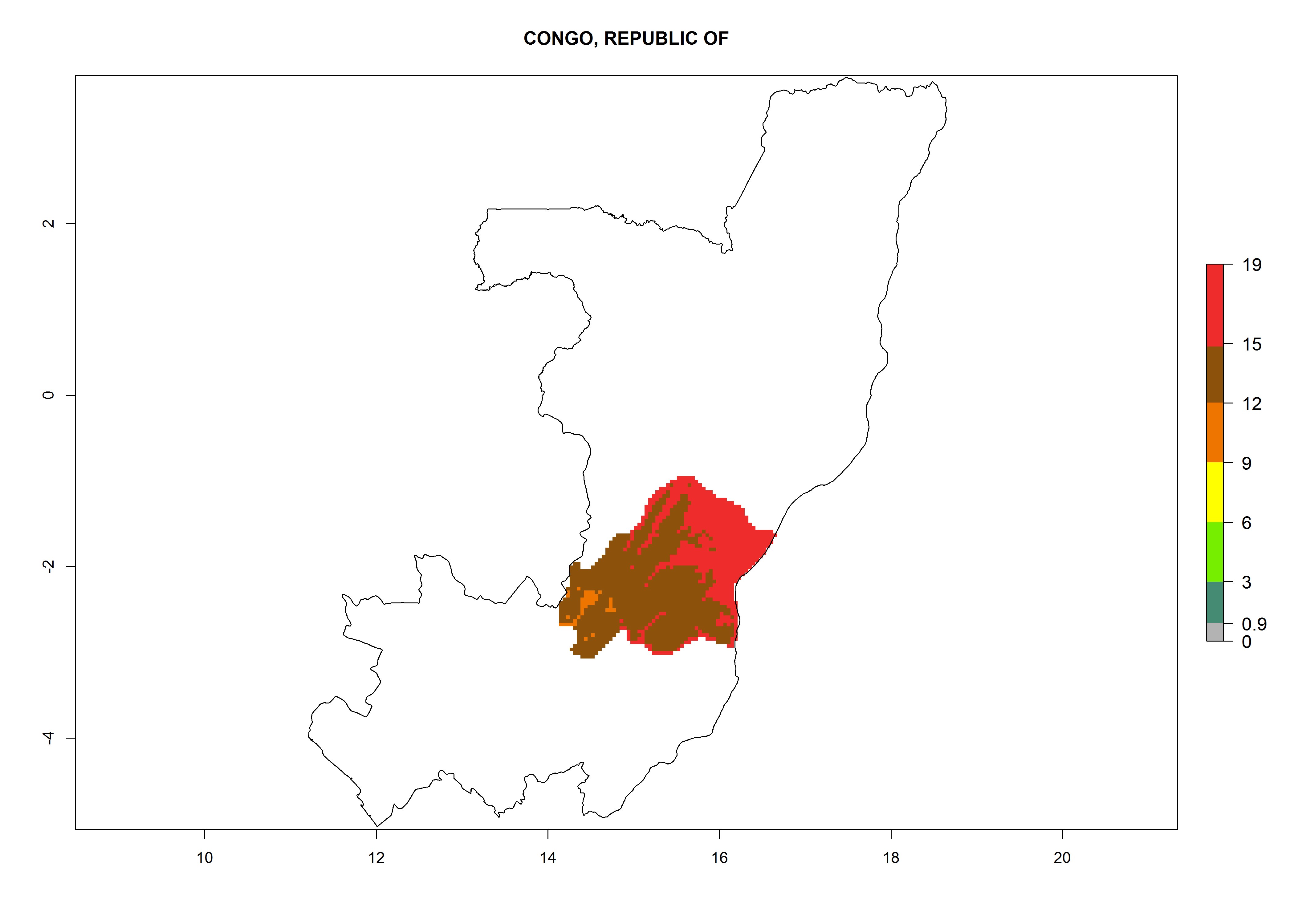
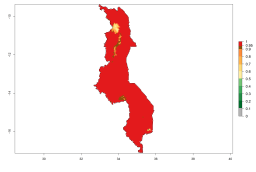
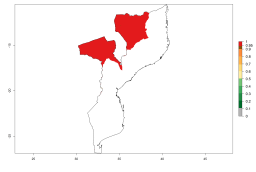
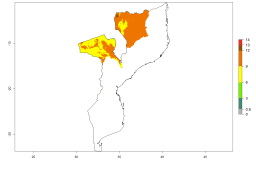
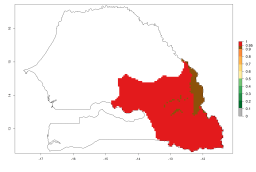
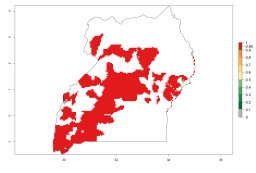
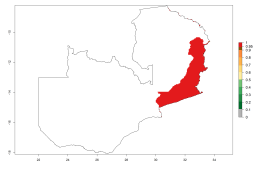
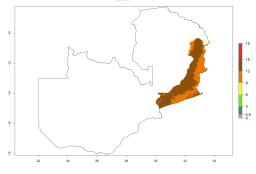
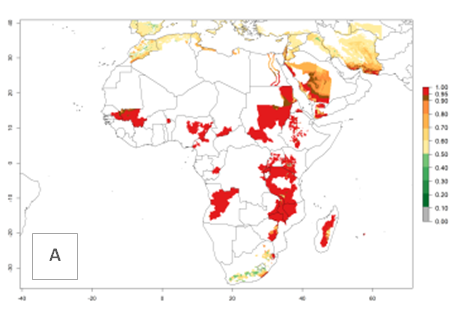
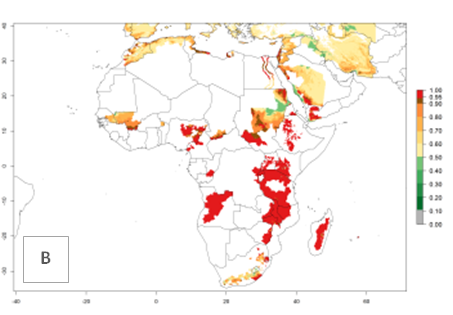
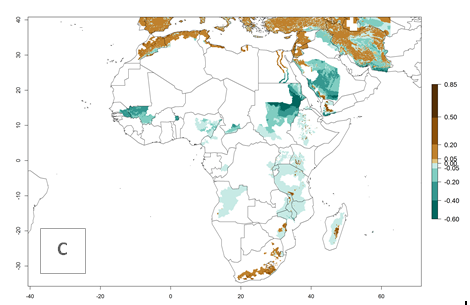
In Africa, A. subandinus has been introduced and established in Zimbabwe, South Africa, Zambia, and Madagascar. And although it has also been introduced into Nigeria and Tanzania, its establishment was not further confirmed. According to the mapping results, however, a successful establishment could have been expected in both countries (Fig. 5). In some tropical African countries, an EI>0.95 indicates that A. subandinus could establish according to temperature conditions in the potato-growing areas of Senegal, Mali, Nigeria, Cameroon, Chad, Sudan, Congo, Angola, Ethiopia, Uganda, Rwanda, Kenya, Mozambique, Malawi, and Tanzania (Fig. 5A). Owing to climate change, it can be expected that the establishment will potentially increase in some other regions of Kenya, South Africa, and Madagascar. In contrast, the establishment will potentially decrease in some regions of Senegal, Mali, Nigeria, Chad, and Sudan (Fig. 5B, C).
 |
 |
 |
|
Figure 5. Changes in establishment and potential distribution of Apanteles subandinus in Africa according to model predictions, using the EI for the years 2000 (A) and 2050 (B) and changes of the EI between 2000 and 2050 (C), displayed in potato production regions with an ERI>0.7 of its primary host Phthorimaea operculella. An EI>0.5 indicates regions with permanent establishment.
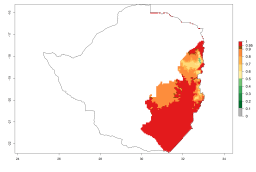
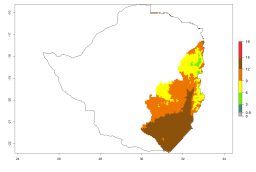
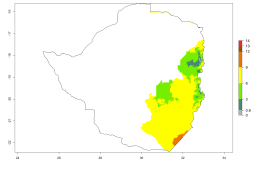
Changes in regional abundance in Africa
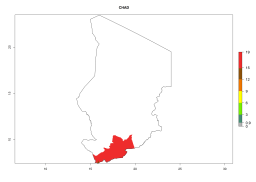
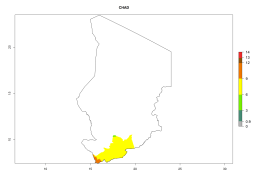
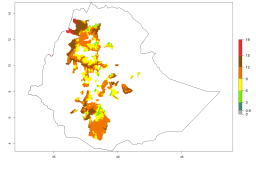
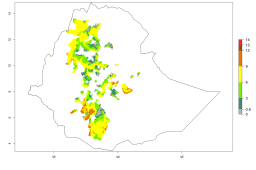
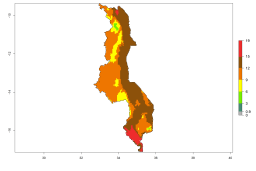
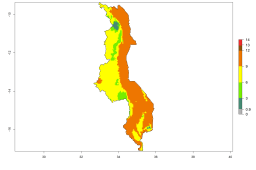
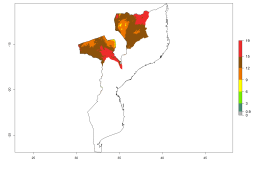
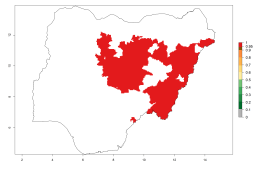
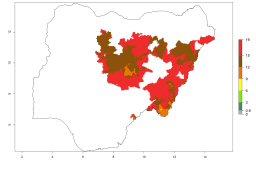
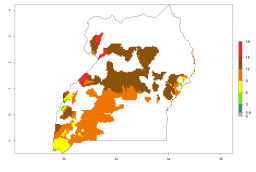
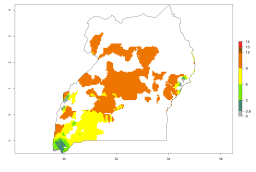
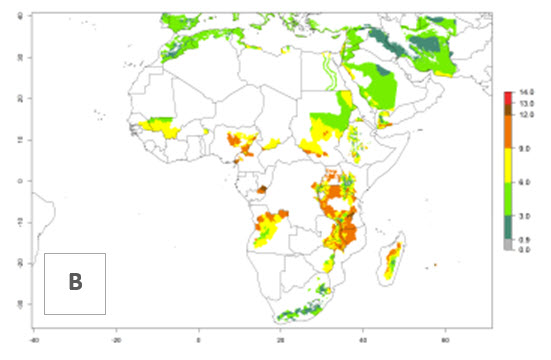
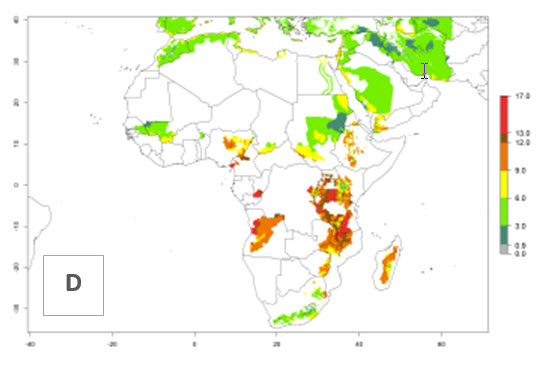
In many potato-growing areas of Africa, a GI>9–15 generations per year have been estimated (Fig. 6C). Predictions for the 2050 climate change scenario show an increase in generation numbers per year in almost all potato-growing areas from West and East Africa (Fig. 6D). The GI is strongly correlated with the AI. The activity is expected to increase in some potato-growing areas of Africa, especially in Congo, Angola, Ethiopia, Uganda, Kenya, Tanzania, Mozambique, Malawi, Zimbabwe, and Madagascar, where a high A. subandinus activity of AI>9 is projected (Fig. 6E and F).
| GI | AI | |
| 2000 | 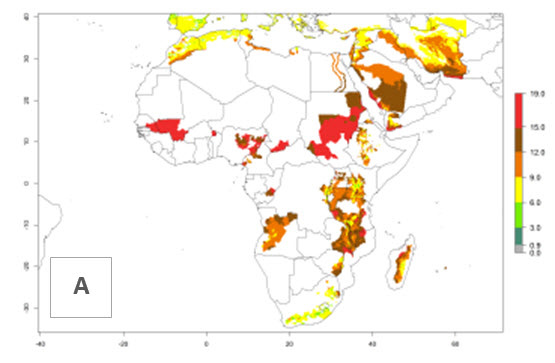 |
 |
| 2050 | 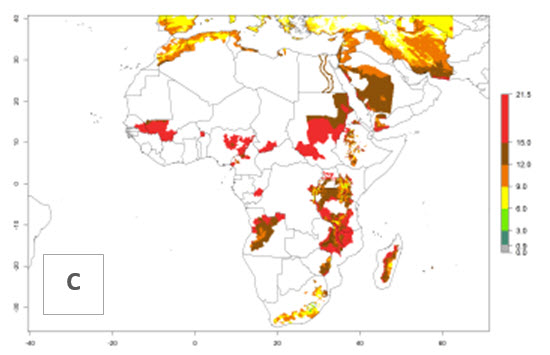 |
 |
| Index change (2000 – 2050) | 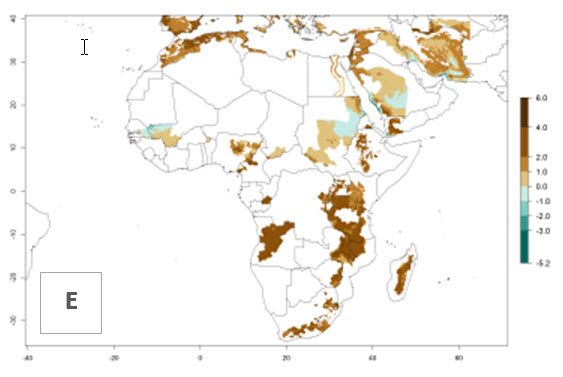 |
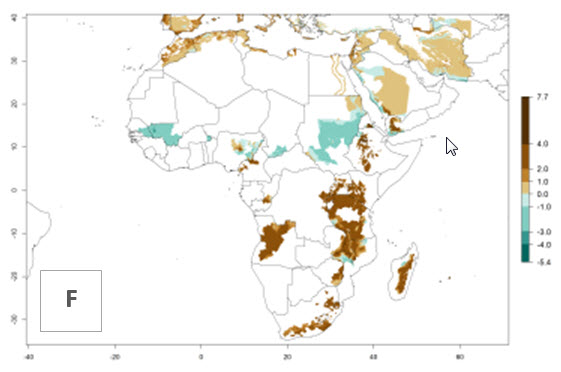 |
Figure 6. Changes in abundance (GI, number of generations/year) and activity (AI, potential population growth) of Apanteles subandinus in Africa according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F), displayed in potato production regions with an ERI>0.7 of its primary host Phthorimaea operculella.
Potential release areas in Africa
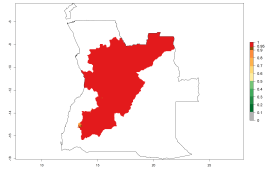
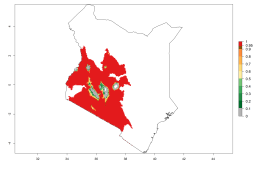
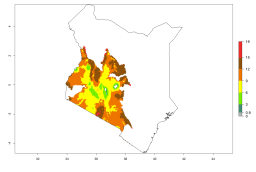
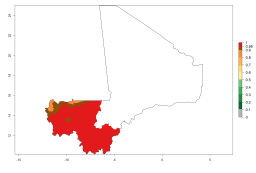
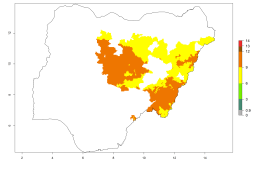
Considering an EI>0.7 for P. operculella in potato-growing areas in Africa, potential countries to release A. subandinus under the present climate are Angola, Cameroon, Chad, Congo, Ethiopia, Kenya, Madagascar, Malawi, Mali, Mozambique, Nigeria, Rwanda, Senegal, South Africa, Sudan, Tanzania, Uganda, Zambia, and Zimbabwe. A. subandinus has been shown to establish at an EI>0.5; in all suggested countries the likelihood of establishment is expected to be higher in almost all potato production regions (EI>0.8). This high establishment potential is associated with a GI>9 (i.e., more than 9 generations develop per year) and an AI>6 (Fig. 7).
Figure 7. EI, abundance (GI, number of generations/year), and activity (AI, potential population growth) of Apanteles subandinus in selected African countries according to model predictions for the year 2000, displayed in potato production regions with an ERI>0.7 of its primary host Phthorimaea operculella. An EI>0.5 indicates regions with permanent establishment.
Risks to non-targets
No risks are reported, although A. subandinus might dominate local parasitoids because of its higher specificity to the potato tuber moth. It is an excellent candidate to be used in the classical biological control of the potato tuber moth.
Further reading
Callan, E.M. 1974. Changing status of the parasites of potato tuber moth Phthorimaea operculella (Lepidoptera: Gelechiidae) in Australia. Entomophaga 19: 97–101.
Dávila, P.W. 2011. Influencia de la temperatura sobre el ciclo biológico de Apanteles subandinus Blanchard (Hymenoptera: Braconidae), parasitoide de Phthorimaea operculella Zeller. BSc thesis, Universidad Nacional Federico Villarreal, Lima, Peru.
Franzmann, B.A. 1980. Parasitism of Phthorimaea operculella (Lep.: Gelechiidae) larvae in Queensland. Entomophaga 25: 369–372.
Horne, P.A. 1990. The influence of introduced parasitoids on the potato moth, Phthorimaea operculella (Lepidoptera: Gelechiidae) in Victoria, Australia. Bulletin of Entomological Research 80: 159–163.
Kroschel, J., and B. Schaub. 2012. Biology and Ecology of Potato Tuber Moths as Major Pests of Potato. In Insect Pests of Potato: Biology and Management, P. Giordanengo, C. Vincent, and A. Alyokhin, eds., 165–192. Elsevier.
Mitchell, B.L. 1978. The biological control of potato tuber moth Phthorimaea operculella (Zeller) in Rhodesia. Rhodesia Agricultural Journal 75: 55–58.
Rao, V., and H. Nagaraja. 1968. Morphological differences between Apanteles scutellaris Muesebeck and A. subandinus Blanchard, parasites of the potato tubermoth, Gnorimoschema operculella (Zeller). Technical Bulletin Commonwealth Institute of Biological Control 10: 57–65.
Salehi, L. 1998. Factors influencing the efficiency of two parasitoids of the potato tuber moth (PTM). Department of Crop Protection, Faculty of Agriculture and Natural Resource Science. University of Adelaide, Adelaide, Australia. pp. 230.
Salehi, L. 2003. A study on reproductive capacities and rate in parasitism of two parasitoids of potato tuber moth. Iranian Journal of Agricultural Sciences 34: 231–245.
Salehi, L., and M.A. Keller. 2002. Investigation on host finding behavior of the two parasitoids of potato tuber moth in a flight tunnel. Journal of Agricultural Science and Technology 4: 95–102.
Sankaran, T., and D.J. Girling. 1980. The current status of biological control of the potato tuber moth. Biocontrol News and Information 1: 207–211.