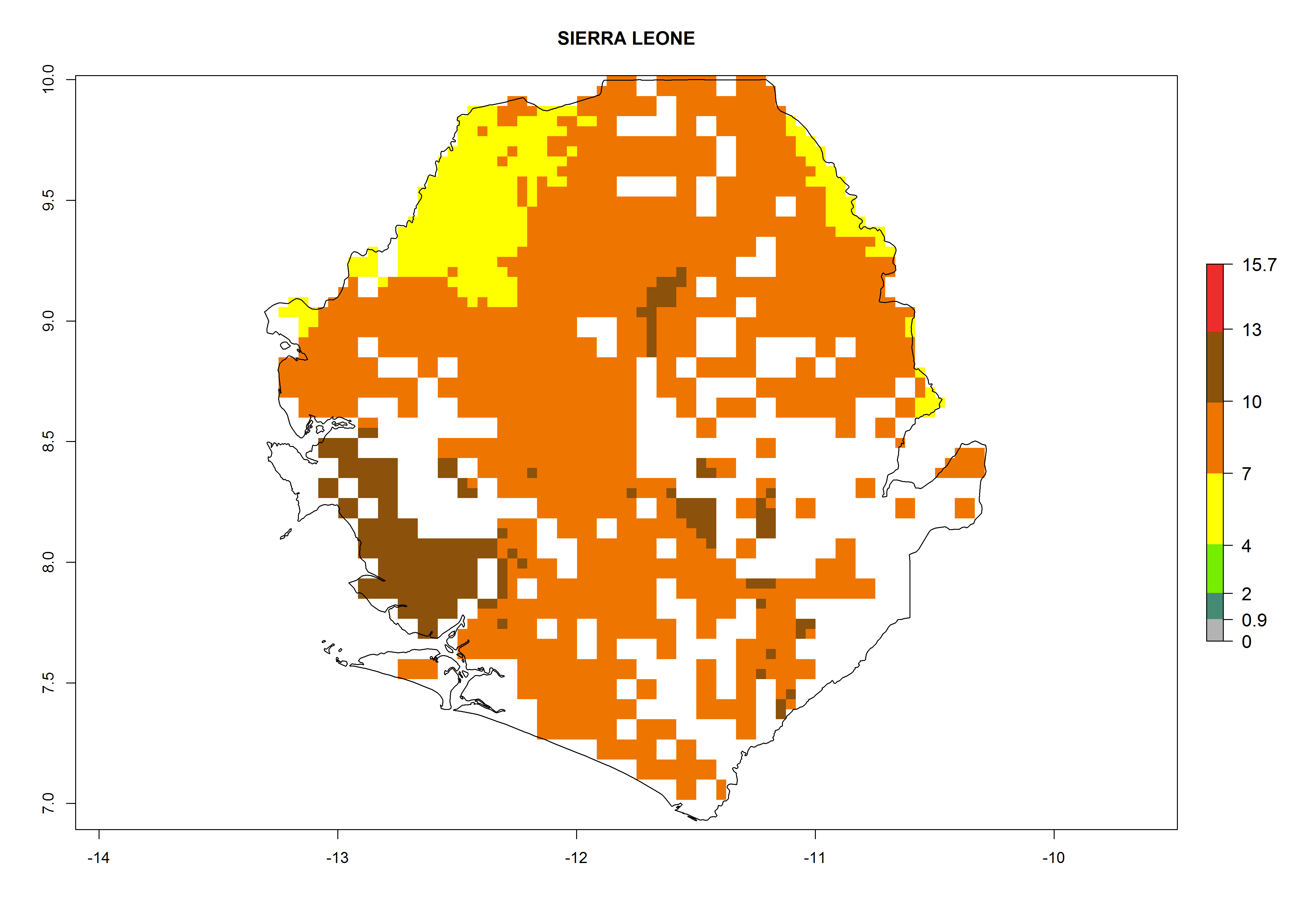4.2.5 Sweetpotato Pests / White fly, Bemisia afer (Priesner & Hosny 1934)![]()
Synonyms: B. hancocki was described from Uganda in 1936.
Taxonomic position: Insecta: Hemiptera: Aleyrodidae
Authors: H. Gamarra, P. Carhuapoma, J. Kreuze, & J. Kroschel
Hosts
Bemisia afer is a polyphagous pest that has been recorded infesting 32 plant families. The major crops and fruit trees are the following: Convolvulaceae: sweetpotato [Ipomoea batatas (L.) Lam]; Euphorbiaceae: cassava (Manihot esculenta Crantz); Fabaceae: beans (Phaseolus vulgaris L.); Lauraceae: laurel (Laurus nobilis L.); Malvaceae: Mexican cotton (Gossypium hirsutum L.), Pima cotton (Gossypium barbadense L.); Myrtaceae: apple guava (Psidium guajava L.); Rutaceae: bitter orange (Citrus aurantium L.), lemon [C. limon (L.) Burnm. F.], sweet orange (C. sinensis Osbeck); Solanaceae: pepino (Solanum muricatum Aiton); and Chinese chili (Capsicum chinense Jacq.). Additionally, some ornamentals are host plants, such as rose mallow (Hibiscus sp.) or rose (Rosa hybrida L.).
Detection and identification
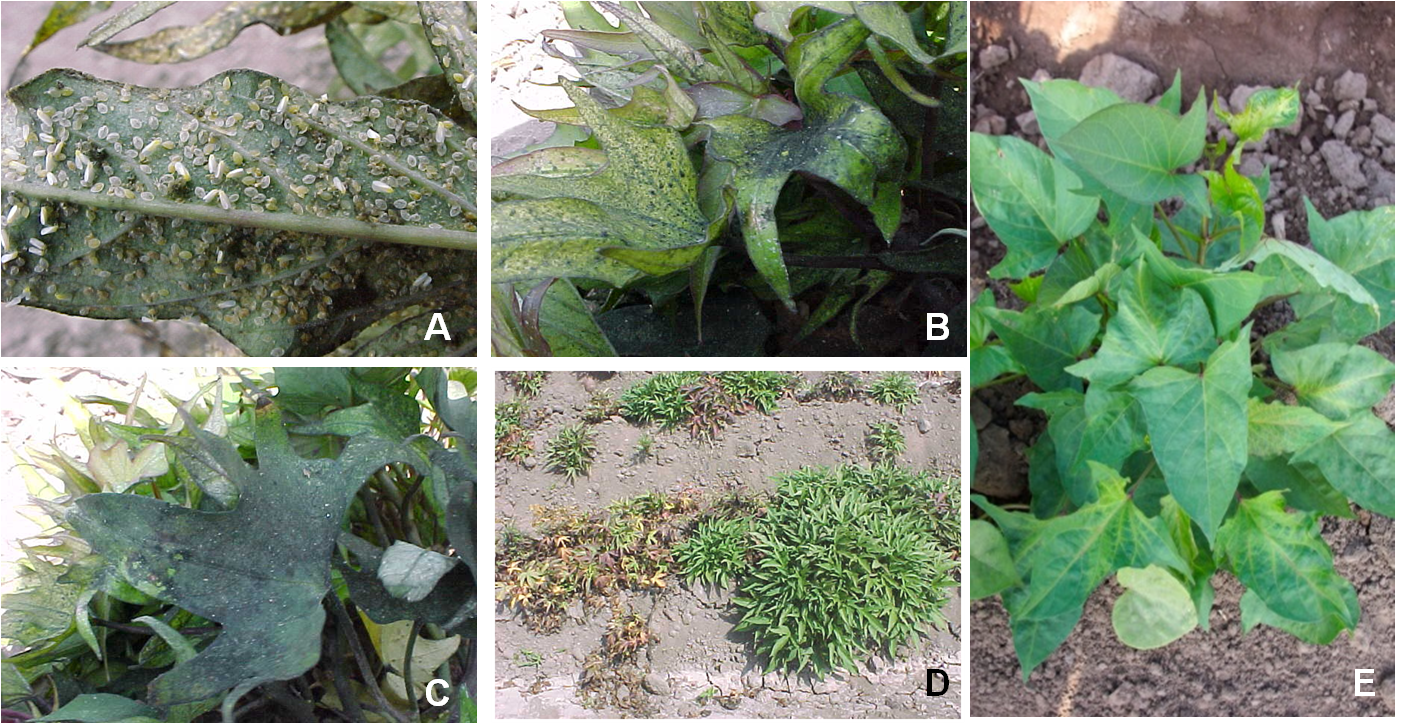
As with B. tabaci (section 4.2.4), the feeding by nymphal instars and adults of B. afer has been associated with several physiological plant disorders. In sweetpotato they can cause severe pod chlorosis, yellowing of young leaves, and sooty mold caused by fungi growing on extensive sugar excretions. The excretions cover and obscure the leaves and interfere with normal photosynthetic processes (Photos 1A–C). Transmission of plant viruses by adults, however, is the most important damage caused by B. afer. Main viruses transmitted are Begomoviruses (Geminiviridae: Begomovirus) in sweetpotato, tomato leaf curl viruses, and cassava and bean mosaic viruses, and viruses of the genera Crinivirus (Family: Closteroviridae) such as the sweet potato chlorotic stunt virus (SPCSV) (Photo 1D). Like B. tabaci, symptoms of virus infections caused by B. afer are not often specific enough to confirm the presence of a particular whitefly species; therefore the use of molecular or morphological studies of the fourth nymphal instar (pupa) are necessary to confirm the identification of the white fly species (see section 4.2.4).
Morphology
Egg
On average, eggs are 0.94 x 1.78 mm in size. Initially they are creamy to transparent (Photo 2A), but as they mature turn to brown and finally black.
Nymphal instars
First nymphal instars (1.60 x 2.63 mm) are called “crawlers”; they are near-transparent, flat, and oval (Photo 2B). Second nymphal instars (2.49 x 4.02 mm) are translucent, oval with scalloped edges, and a yellow-greenish body (Photo 2C). Third nymphal instars are about twice the size of the first nymphal instars (4.49 x 6.69 mm) but morphologically similar to the second nymphal instar (Photo 2D).
Pupa (Fourth nymphal instar)
The fourth nymphal instars are oval (pupal), flat, and almost transparent; like a scale, the dorsal surface occasionally presents clear ratings and good nodule and papillae development. It measures 5.58 x 8.34 mm on average in fully developed pupae. Close to adult emergence, the eyes can be seen (Photo 2E).
Adult
Wings of newly emerged adults (5.35 x 9.51 mm for males, 5.65 x 10.50 mm for females) are clear but in time become covered with a white wax (Photo 2F).
Photos 2. The developmental stages of the whitefly, Bemisia afer: (A) egg, (B and C) I-III nymphal instars, (D) IV nymphal instar (pupa), and (E) adult. Photos: Courtesy of CIP.
Biology
The whitefly B. afer is a small plant-feeding insect with piercing-sucking mouthparts. Both immature and adult whiteflies feed on the undersides of leaves. Females usually lay their eggs in small groups, which are sometimes circular or semicircular as a result of the rotation of the female around her feeding stiletto. The first nymphal instar (crawler) is mobile with tiny legs for moving across the leaf surface. After hatching it may travel a short distance until it successfully finds a suitable position to obtain sap, where it then settles to feed. The following developmental stages are sessile and do not move from the feeding site originally selected by the crawler. The pupa is considered the last non-feeding stage of the fourth instar. Adult whiteflies emerge through a T-shaped slit in the integument of the last nymphal instar. Mated females may live up to 41 days, whereas adult males do not live more than 23 days. Females lay 70–400 eggs shortly after emergence but may also have a pre-oviposition period of 1 day
Temperature-dependent development
B. afer successfully developed from egg to adult at temperatures of 17º–28ºC but failed at 10°C and 13ºC, as no nymphs developed at these temperatures (see Annex 7.3.8). The total mean development time decreased with increasing temperature, from 40.8 days at 17°C to 29.8 days at 25°C, and then increased slightly to 35.1 days at 28ºC. The minimum mortality for egg (1.2–4.7%), nymph (10.1–27.6%), and pupae (0–2.2%) ranged 17º–25ºC. The mean longevity significantly decreased with increasing temperature, with 8.7 and 10.2 days at 17ºC and 23.6 and 22 days at 28ºC for females and males, respectively. The maximum fecundity of 135 eggs was observed at 25ºC.
The models established to describe the development time, survivorship, and reproduction were compiled into an overall stochastic phenology model that allows the B. afer life-table parameters to be estimated according to temperature (see Annex 7.3.8). The predicted intrinsic rates of natural increase (rm) indicate that populations develop within the range of 18º–27ºC. The negative r values below 18ºC and above 27ºC indicate that population size is decreasing due to high mortality or very low reproduction. The highest population growth can be expected at 23ºC (rm = 0.0983). Similarly, finite rate of increase for white fly peaked at 23ºC (λ = 1.1034) and was smallest when exposed to <16ºC and >27°C; λ values of <1 indicate that the population is decreasing. At 23ºC doubling time (Dt) was shortest with 7.05 days. The highest values for reproductive parameters were at 23ºC for the gross reproductive rate, with 43.03 female offspring/female. When mortality of females before reproduction was considered, the net reproductive rate (Ro) was highest at 22.5ºC, with around 26.4 female offspring/female. The mean generation time (T) decreased with increasing temperature and was shortest at 25ºC, with 31.9 days from egg to egg. These simulations indicate that B. afer is able to establish in most tropical production areas of the world.
Deterministic simulation of life-table parameters under temperature conditions of the lowland agro-ecology in Peru (La Molina: 12º05’S, 76º57’W, 250 masl, mean annual precipitation of 6.4 mm, mean temperature of 19.7ºC) showed that B. afer population increases in all seasons. According to T, 8.8 generations can develop per year under these environmental conditions and, during the different seasons—winter, autumn, spring, and summer, respectively—can develop the following generations: 1.6 (mean temperature: 16.3ºC), 2.3 (mean temperature: 18.8ºC), 1.8 (mean temperature: 19ºC), and 2.5 (mean temperature: 23.6ºC).
Means of movement and dispersal
Adults of B. afer do not fly very effectively, but once airborne, they can be transported by wind over quite large distances. Similar to B. tabaci (section 4.2.4) and T. vaporariorum (section 4.3.4), its activity is mostly during the day with little movement at night. Long-distance movement and transportation are through infestations of planting material or cut flowers.
Economic impact
B.afer can damage plants in different ways: it can cause direct damage through reduction of plant vigor, product quality, and yield, as reported on the Peruvian coast, causing losses in sweetpotato. However, it may also cause indirect damage through transmission of viruses in crops of economic importance. In Africa, B. afer and B. tabaci (Gennadius) have been found together on cassava plants. B. afer is considered a potential vector of Cassava brown streak virus, which mixed populations of B. afer and B. tabaci were recently found to transmit successfully. However, B. afer has only been confirmed as a vector of SPCSV, the most important virus of sweetpotato worldwide, due to its ability to mediate severe synergistic diseases with several other sweet potato-infecting viruses belonging to different genera. Yield reductions caused by SPCSV are approximately 30% but can exceed 90% when interacting with other viruses.
Geographical distribution (permanent establishment)
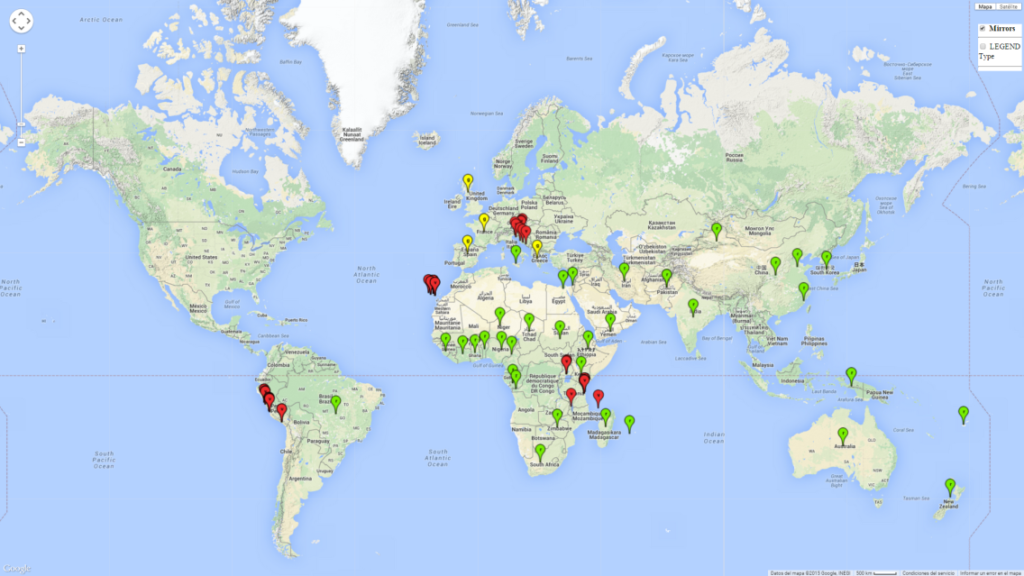
Broadly speaking, B. afer has become a global pest, with records in each geographical region of the world (Fig. 1). Under field conditions, it infests many crops and wild hosts in tropical, subtropical, and temperate zones of the world, including Africa, Oceania, the Mediterranean coast of Europe, southern England, Asia, and South America. It is not native to Europe, but has become successfully established in glasshouses and, in some countries, in the field.
| Africa | Benin, Cameroon, Chad, Congo, DR Congo, Comoro Islands, Ivory Coast, Egypt, Ethiopia, Ghana, Kenya, Malawi, Madagascar, Nigeria, Niger, Reunion, Rhodesia, Sierra Leone, South Africa, Sudan, Tanzania, Uganda |
| Asia | China, India, Iran, New Guinea, Pakistan, Yemen, South Korea |
| South America | Peru, Brazil |
| Europe | Croatia, Greece (greenhouse), Italy (Sicily), Fiji, Spain (Canary Islands), Tonga, United Kingdom (greenhouse) |
| Oceania | Australia |
Phytosanitary risks
B. afer is found worldwide. Nevertheless, recent molecular evidence suggests that B. afer, like B. tabaci, consists of a species complex. It may be that different “biotypes” that vary in biological properties are only locally common. Although there is no direct evidence for this yet, a risk exists that variants of B. afer with novel properties are introduced into new regions or areas where they were not previously present (e.g., through infested planting materials).
Risks mapping under current and future climates
Global Risks
Changes in establishment and future distribution
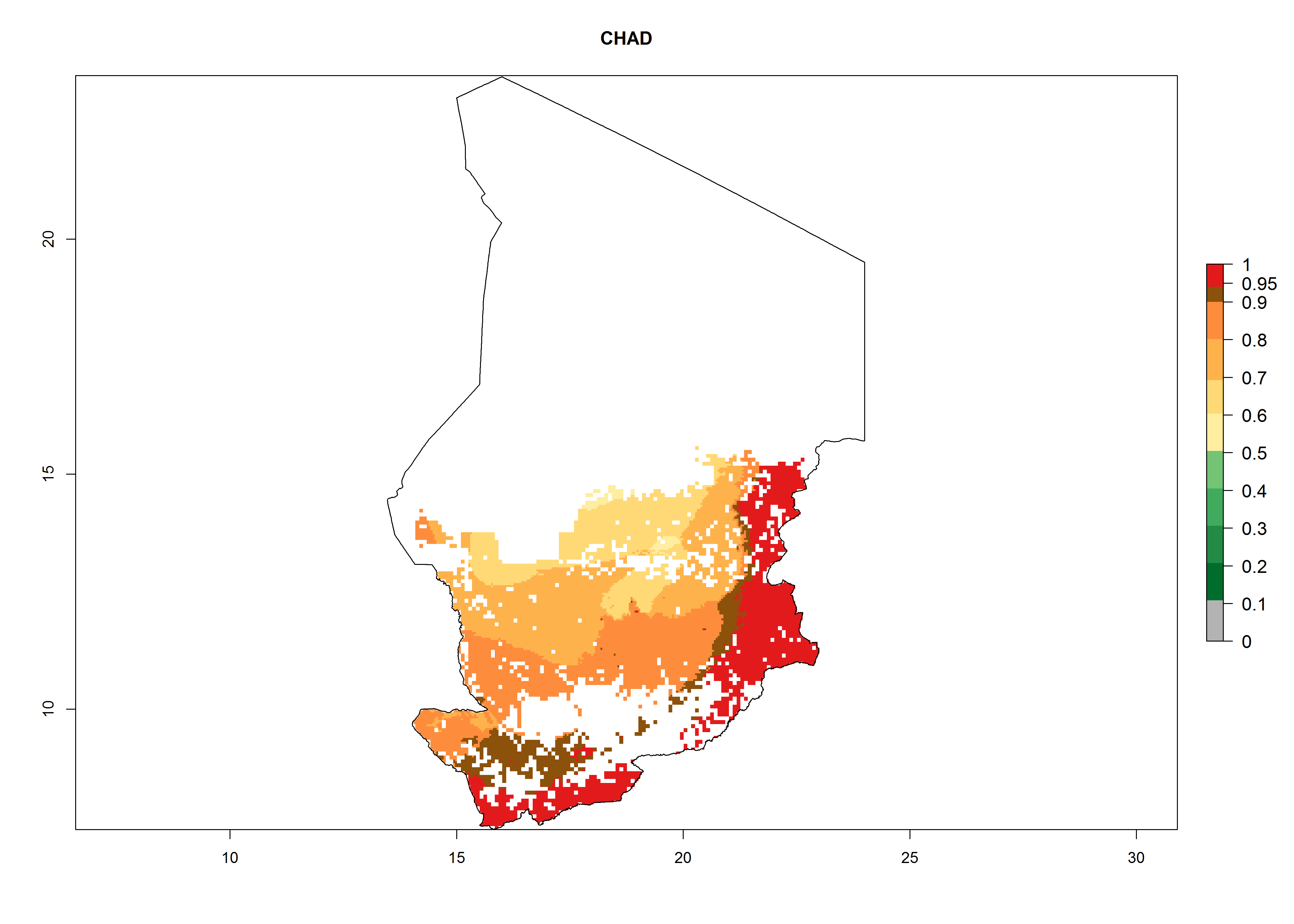
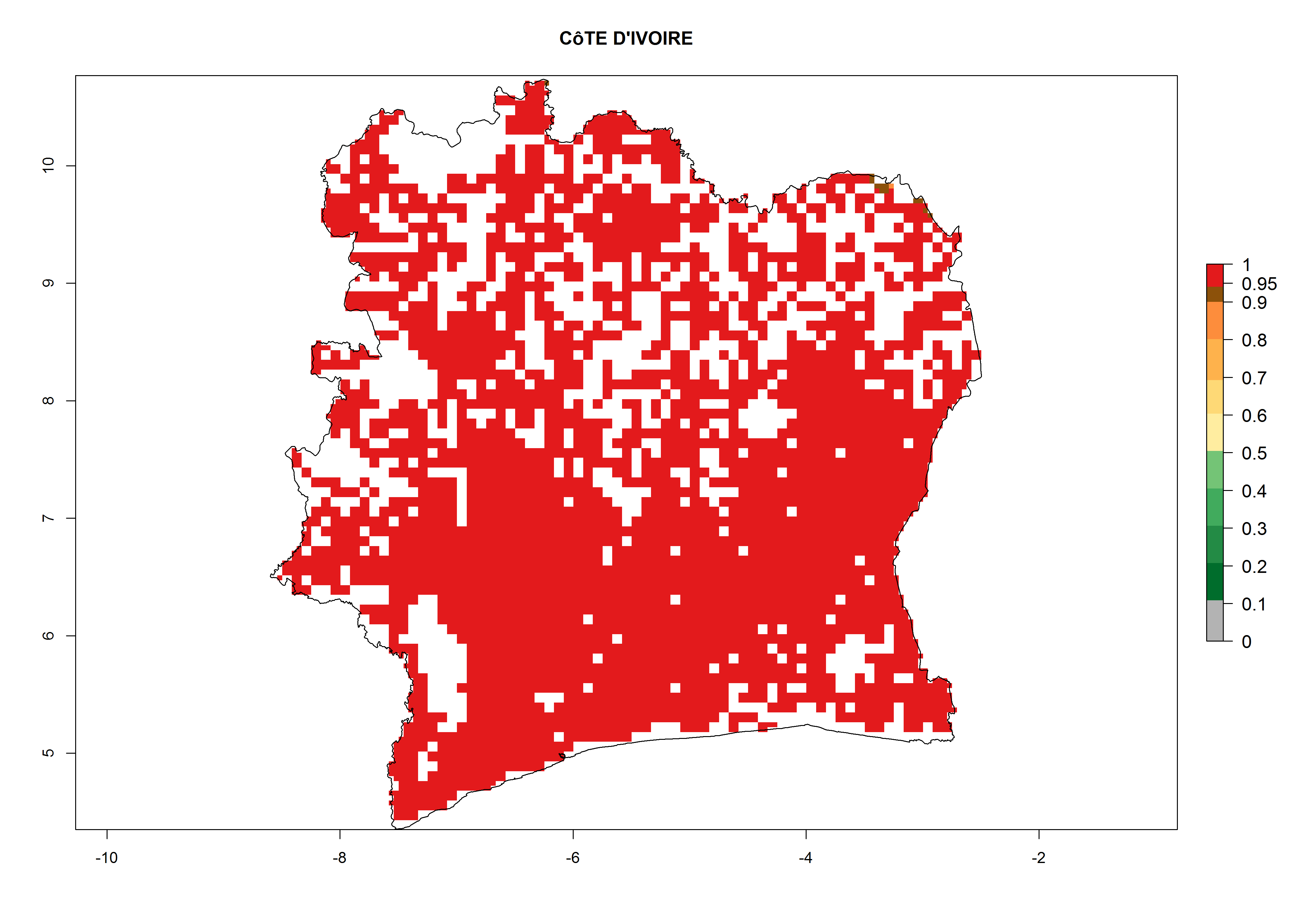
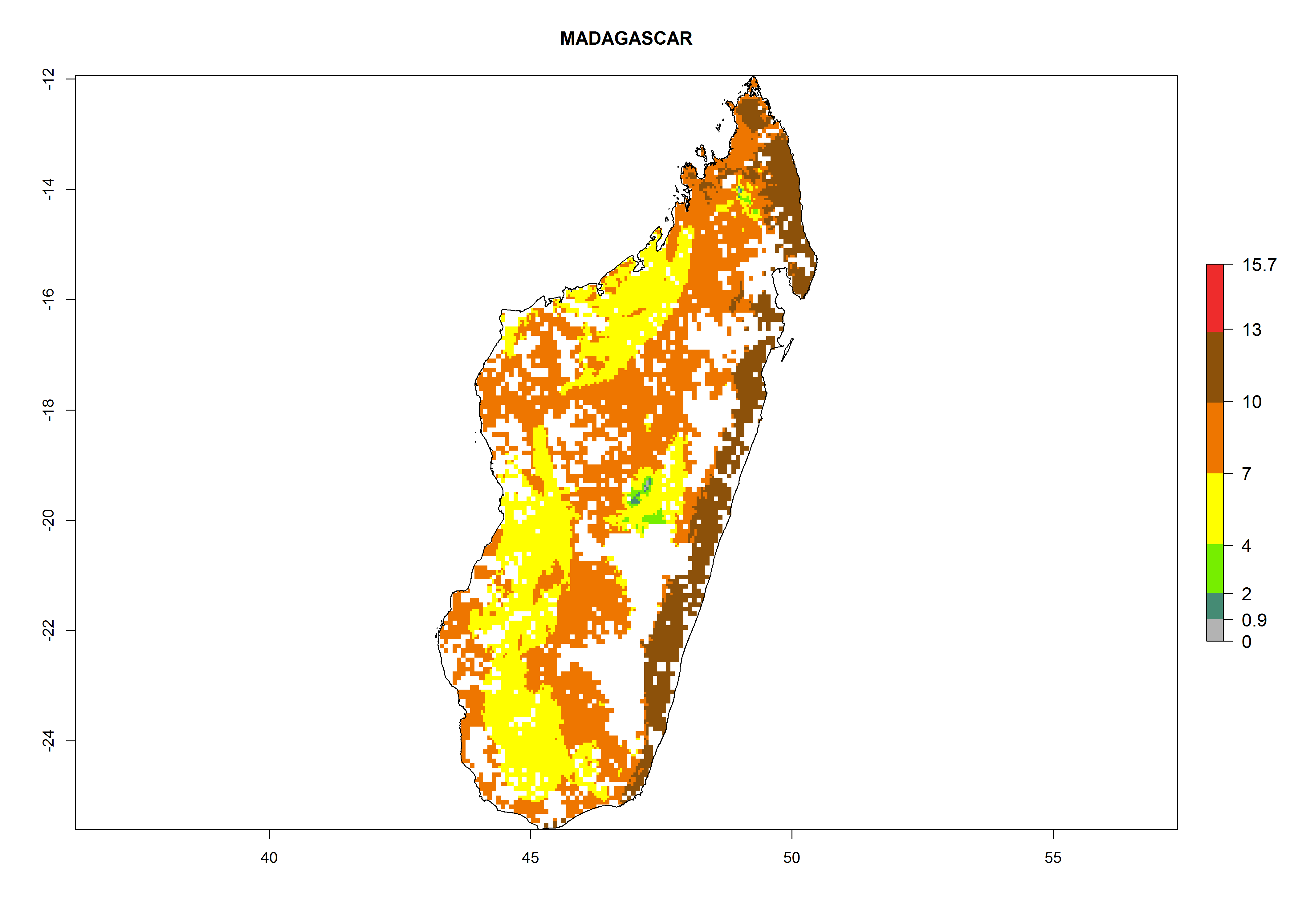
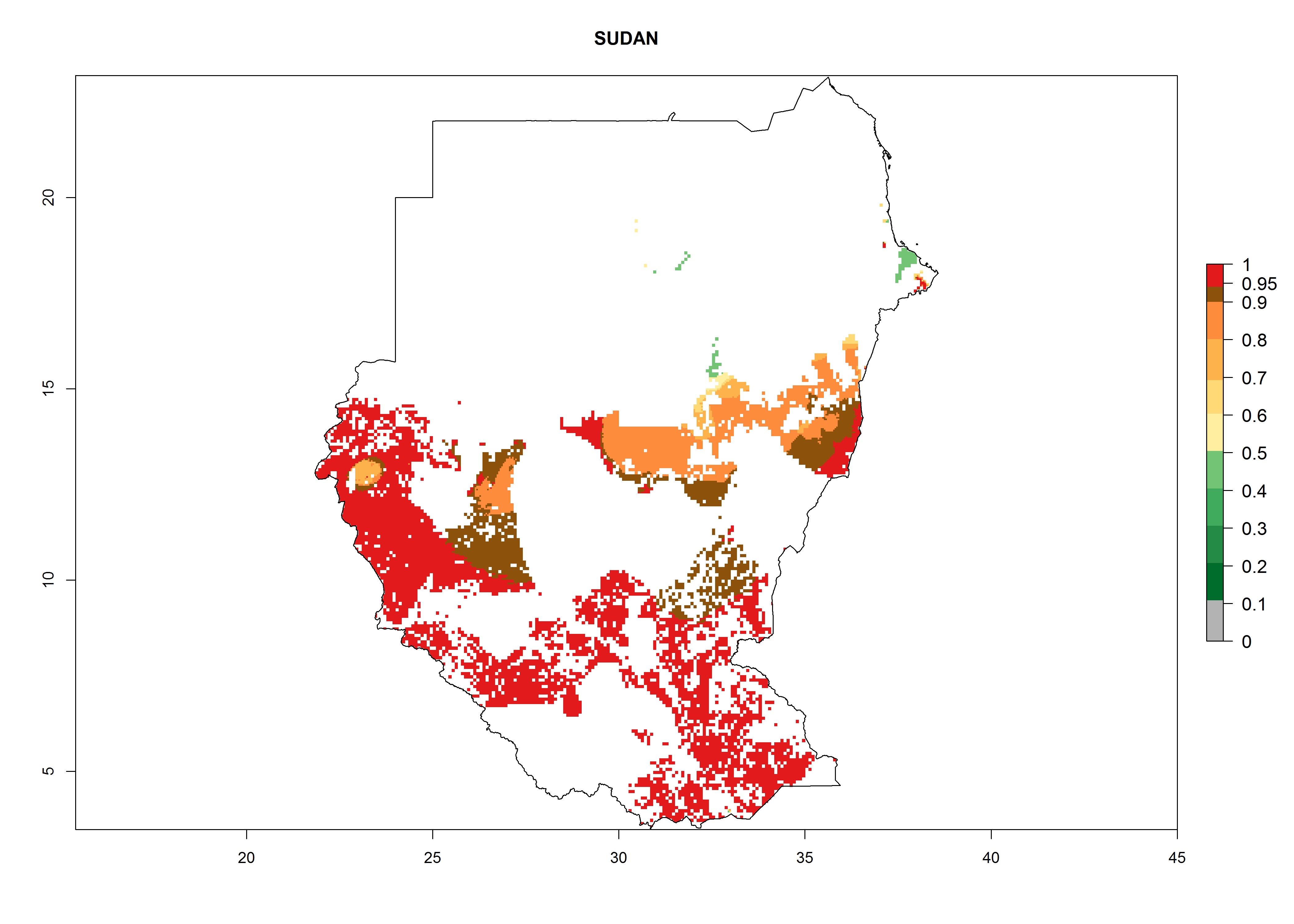
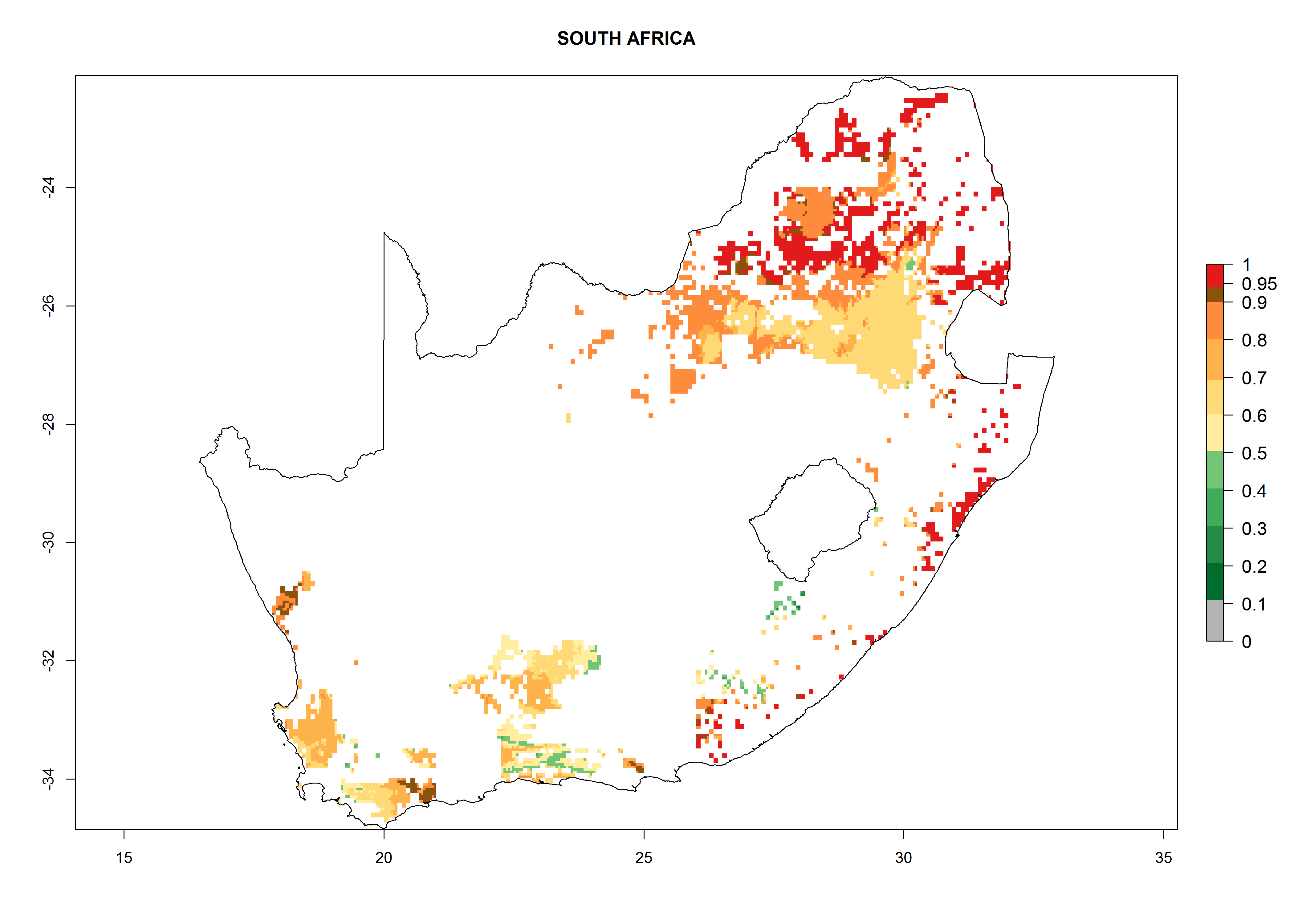
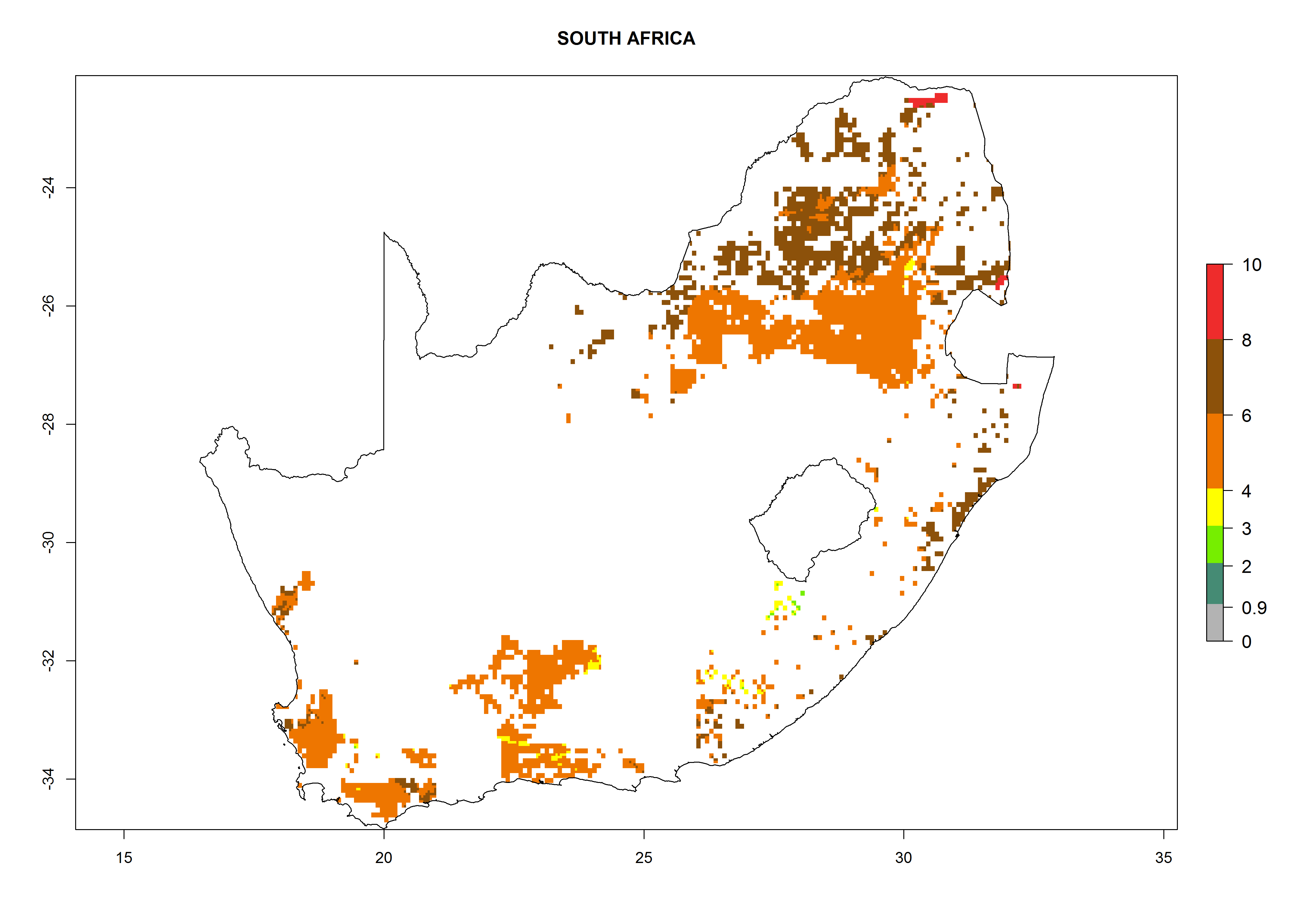
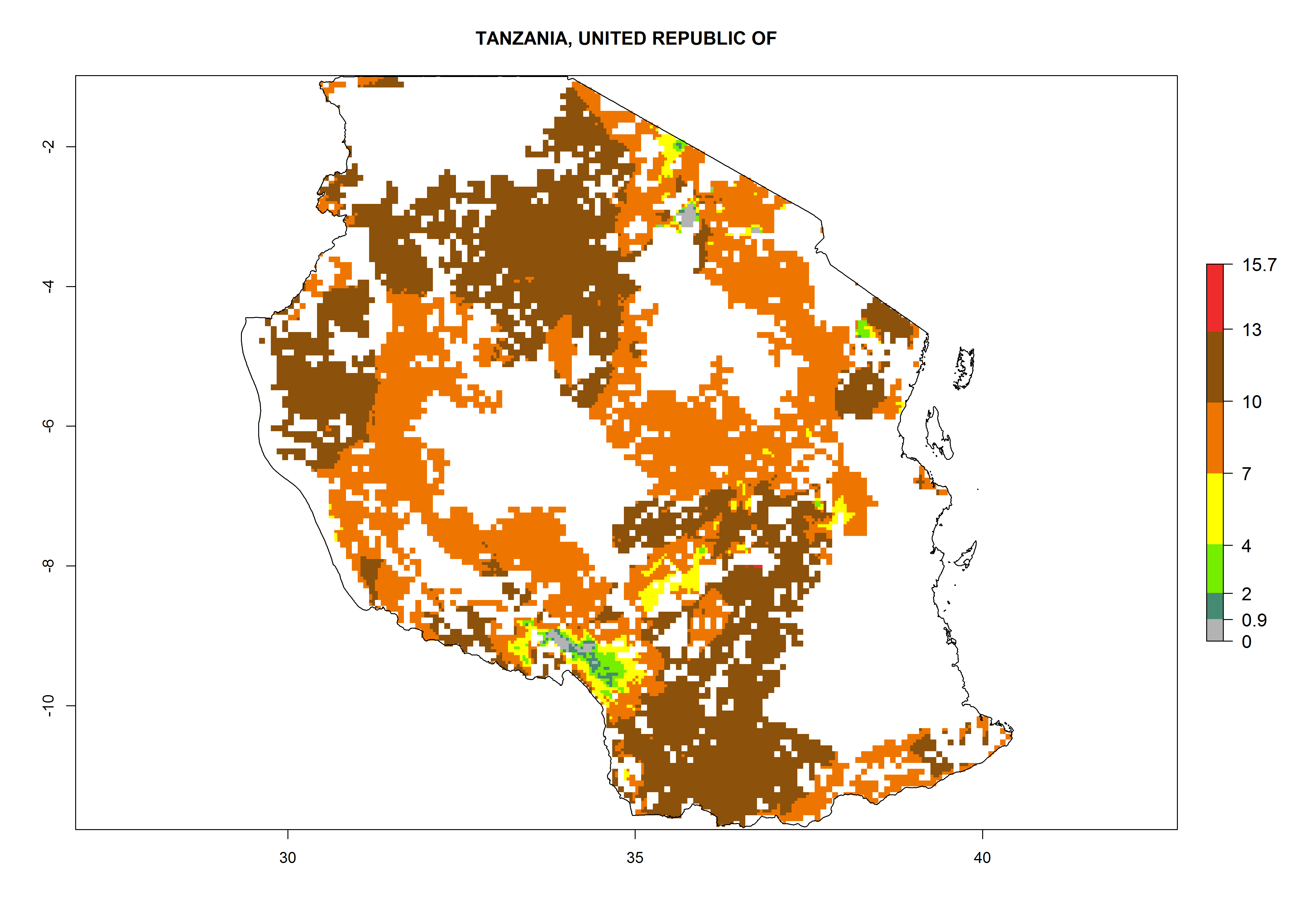
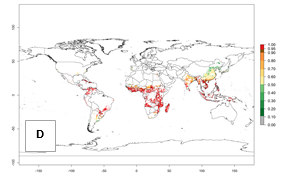
Regions with an establishment risk index (ERI)>0.95 indicate temperature conditions where a certain proportion of the B. afer population is expected to survive throughout the year (Fig. 2A, D). This ERI reflects well the current global distribution of the pest under the climate of the year 2000 in tropical areas of Africa, Asia, Oceania, and South America (compare with Fig. 1). B. afer also occurs in restricted sweetpotato-growing areas with an ERI>0.6–0.9 (dark-yellow zones) as in southern Italy (Sicily), southern China (Fujian), Southern Africa (some areas of South Africa), and Chad (Fig. 2D). B. afer has also been reported in regions with a low likelihood of establishment (ERI<0.6), as in temperate regions of central and northern China (Xinjiang, Shandong, Shaanxi), Italy, South Korea, and Spain, corresponding to infestations in greenhouses and not due to survival under open-field conditions (Fig. 2D). In the year 2050, the predicted risk of B. afer establishment will potentially reduce in current high-risk areas (ERI>0.95) of the tropics globally due to high temperatures (Fig. 2B, C). Instead, in some sweetpotato-growing areas of the subtropics of Argentina, southern Brazil, Uruguay, Peru, Chile, and South Africa, the risk of establishment will increase ERI>0.95 (Fig. 2E, F).
| ERI worldwide | ERI in sweetpotato production systems | |
| 2000 | 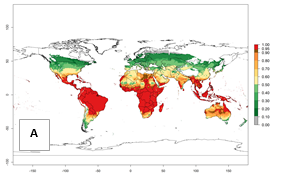 |
 |
| 2050 | 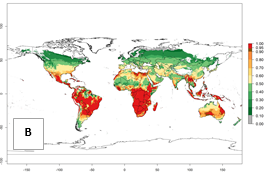 |
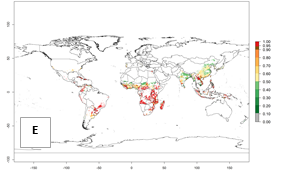 |
| Index change (2000 – 2050) | 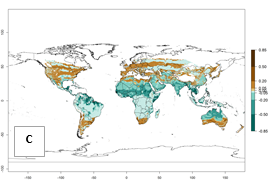 |
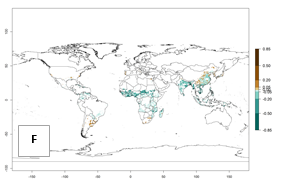 |
Figure 2. Changes in establishment and potential distribution of the whitefly, Bemisia afer, worldwide and in sweetpotato production systems according to model predictions, using the ERI for the years 2000 (A, D) and 2050 (B, E), and changes of the ERI between 2000 and 2050 (C, F). An ERI>0.95 is associated with potential permanent establishment.
Changes in abundance
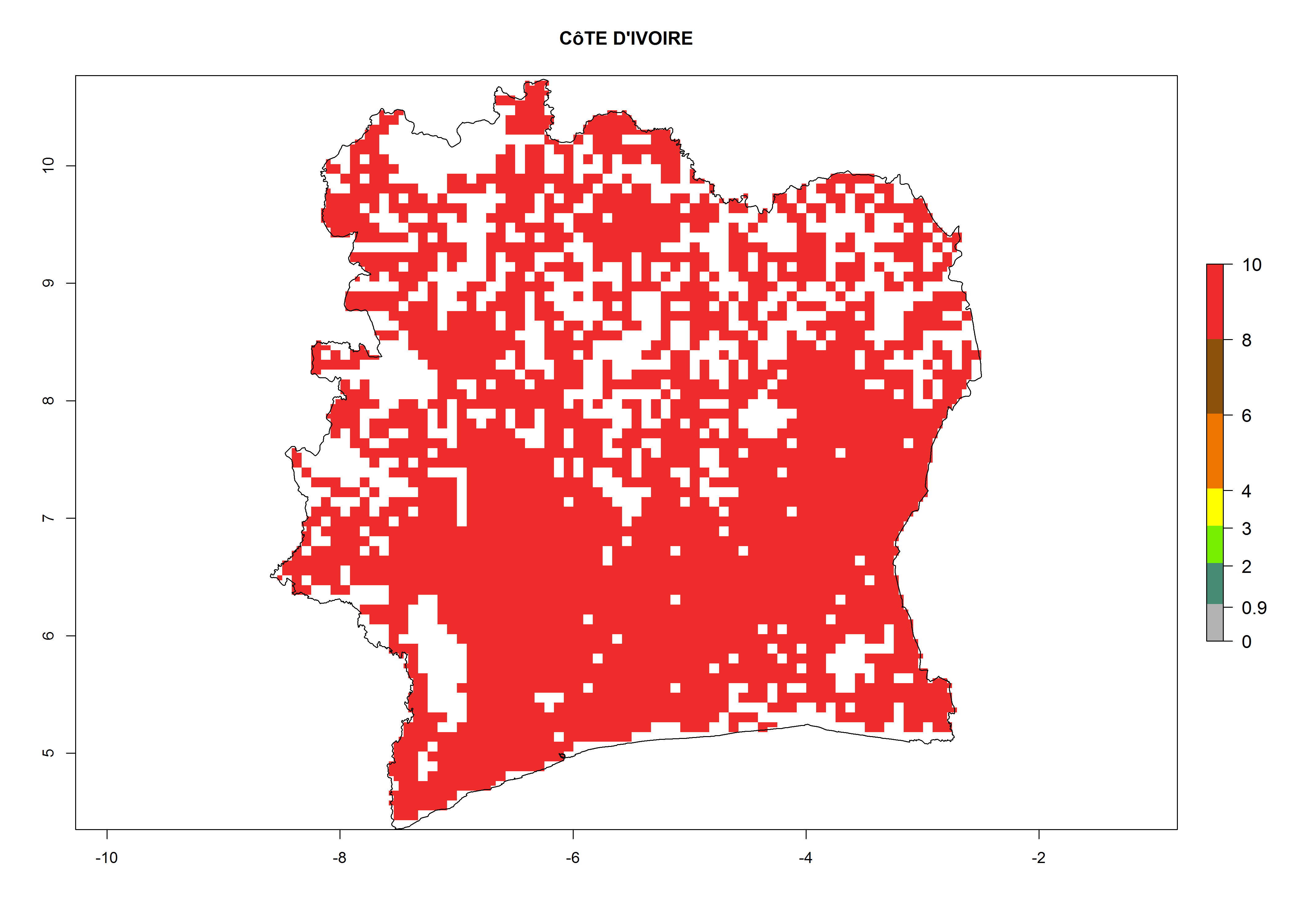
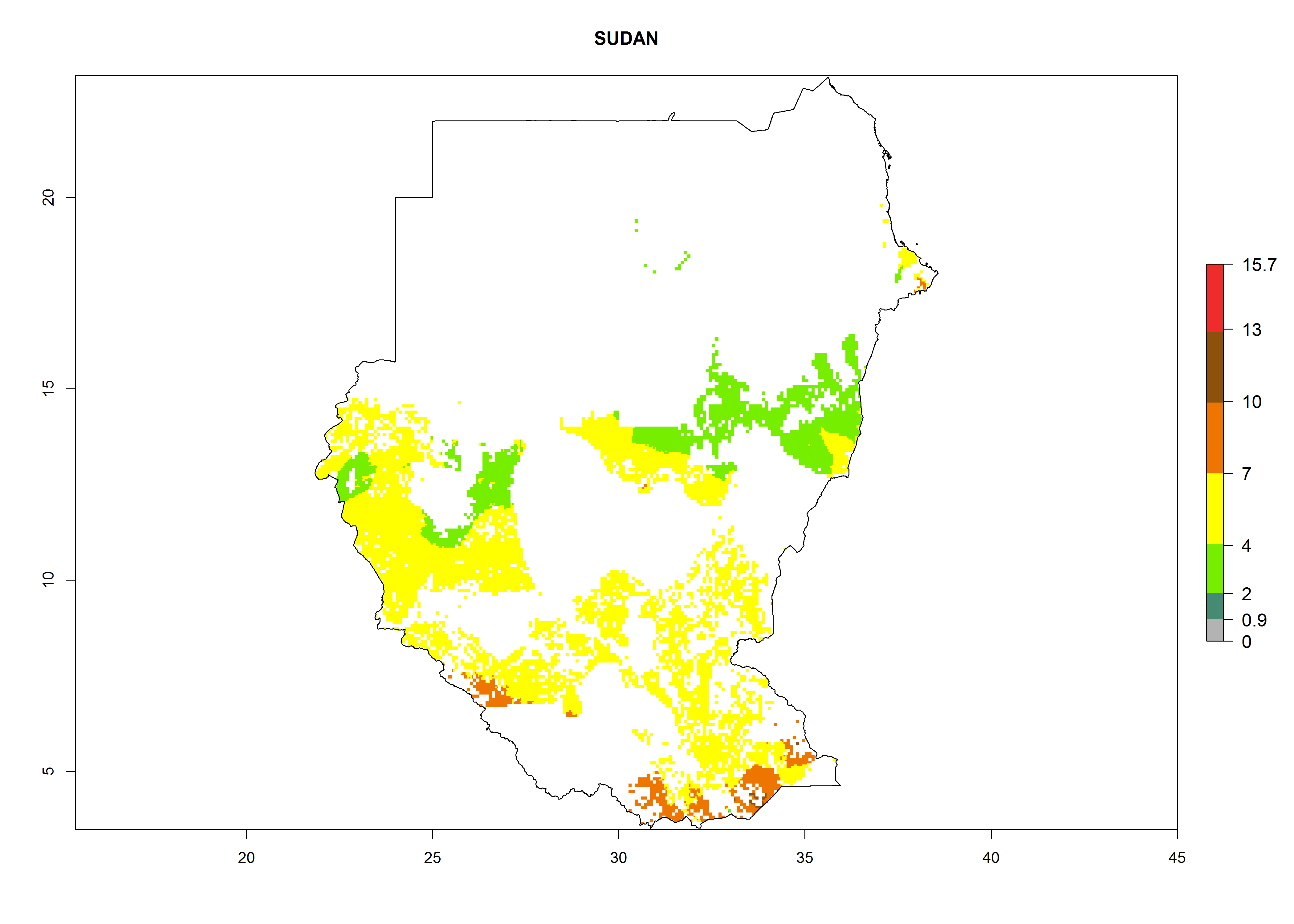
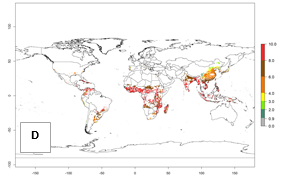
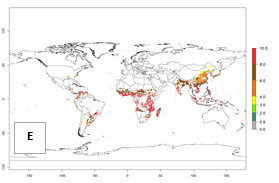
Under current temperature, potentially 8–10 and 4–8 generations per year can develop globally in most tropical and subtropical regions as well as those where sweetpotato is grown (Fig. 3A, D). The generation index (GI) change indicates a slight decrease globally for the year 2015 in most tropical zones. An increase of 1–2 generations per year is forecast for most subtropical regions, including South America (Peruvian coast, central Colombia, southern Brazil); Central, East, and Southern Africa; the Caribbean; Asia (Indonesia, Malaysia, Philippines); southern and central China; Europe (Portugal); and Oceania (Papua New Guinea) (Fig. 3B, E, C, F). In temperate regions of Asia (northern China, Japan, North and South Korea), North America, and European countries, an increase of only 1 generation per year is projected (Fig. 3C, F).
| GI worldwide | GI in sweetpotato production systems | |
| 2000 | 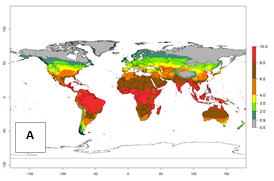 |
 |
| 2050 | 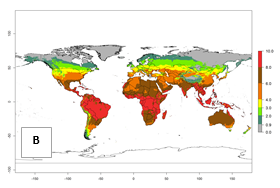 |
 |
| Index change (2000–2050) | 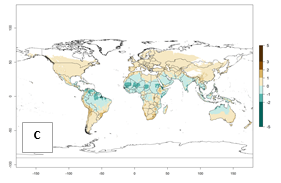 |
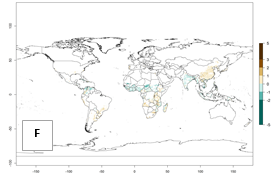 |
Figure 3. Changes in abundance (GI, damage potential) of the whitefly, Bemisia afer, worldwide and in sweetpotato production systems according to model predictions, using the GI for the years 2000 (A, D) and 2050 (B, E), and the absolute index change (C, F).
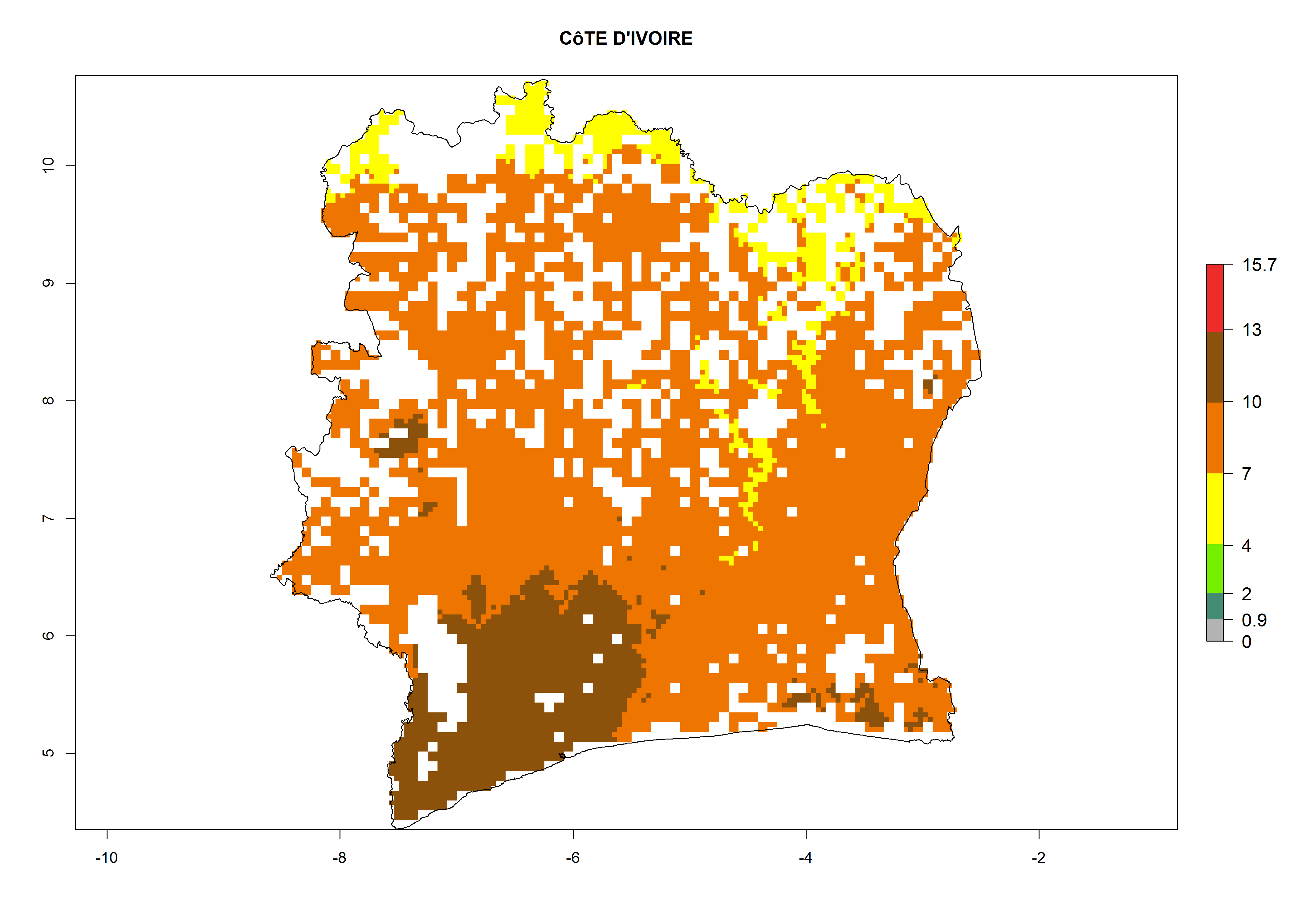
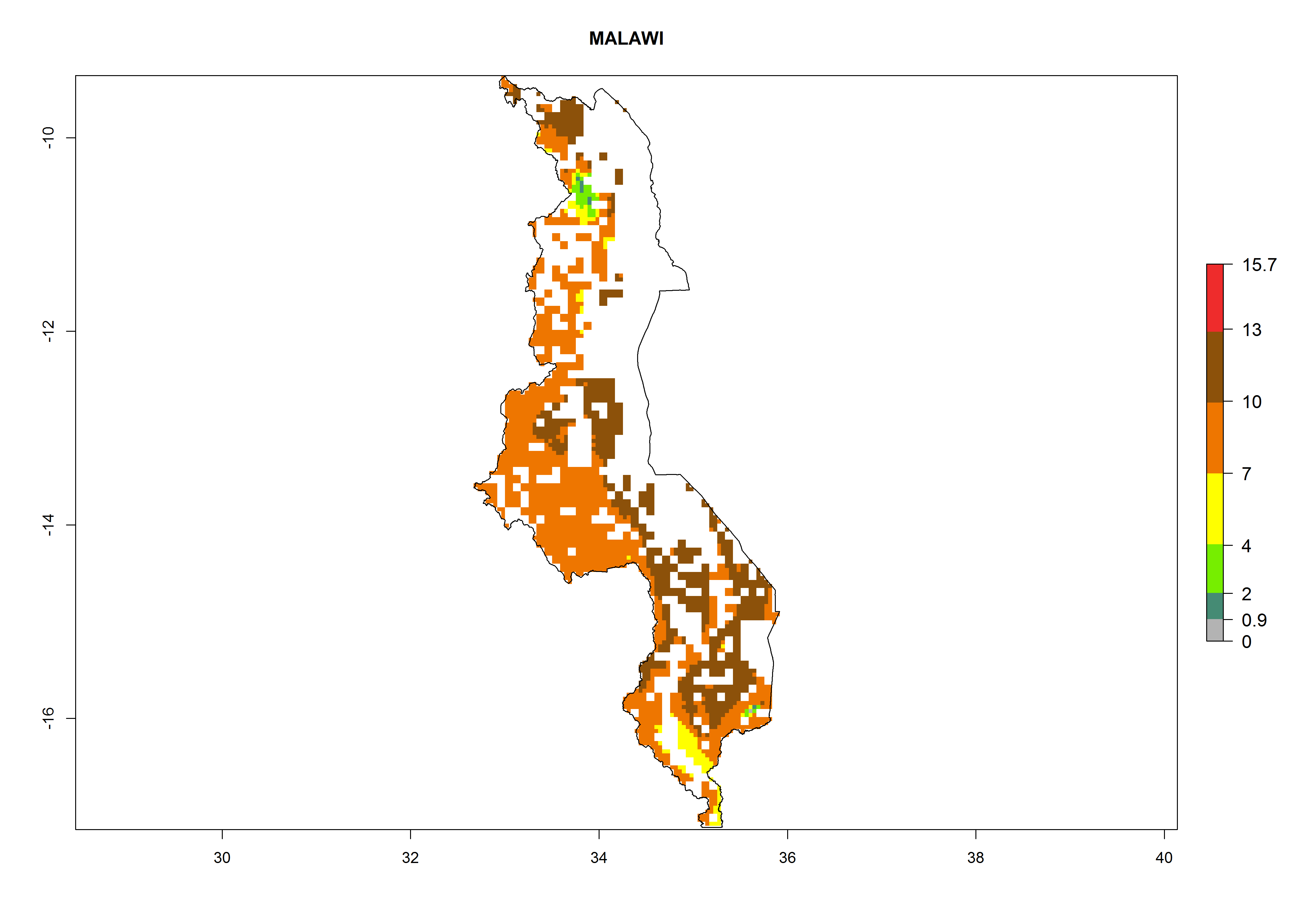
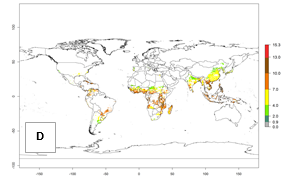
For the year 2000, an activity index (AI)>7–13 indicates a high activity of B. afer in most tropical regions. An AI>2–7 has been estimated for most subtropical regions in North and South America, Asia, Africa, and the Mediterranean region, including the sweetpotato-growing regions (Fig. 4A, D). Predictions of changes for the year 2050 climate change scenario show a decrease in the potential growth of B. afer in most tropical and subtropical sweetpotato-growing regions (Fig. 4B, C, E, F). In southern subtropical as well as temperate zones, B. afer activity will potentially increase (Fig. 4C, F).
| AI worldwide | AI in sweetpotato production systems | |
| 2000 | 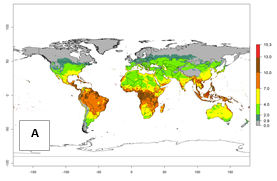 |
 |
| 2050 | 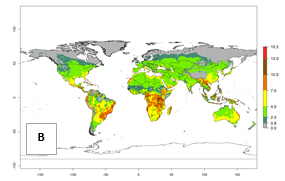 |
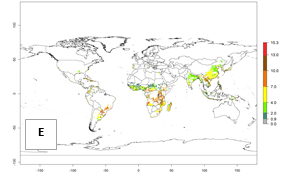 |
| Index change (2000 – 2050) | 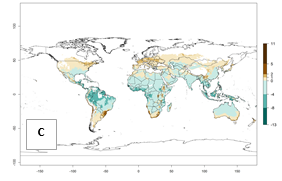 |
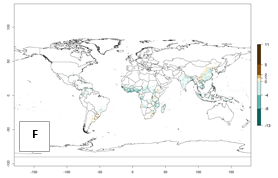 |
Figure 4. Changes in activity (AI, potential population growth) of the whitefly, Bemisia afer, worldwide and in sweetpotato production systems according to model predictions, using the AI for the years 2000 (A, D) and 2050 (B, E), and the absolute index change (C, F).
Regional Risks for Africa
Changes in establishment and future distribution
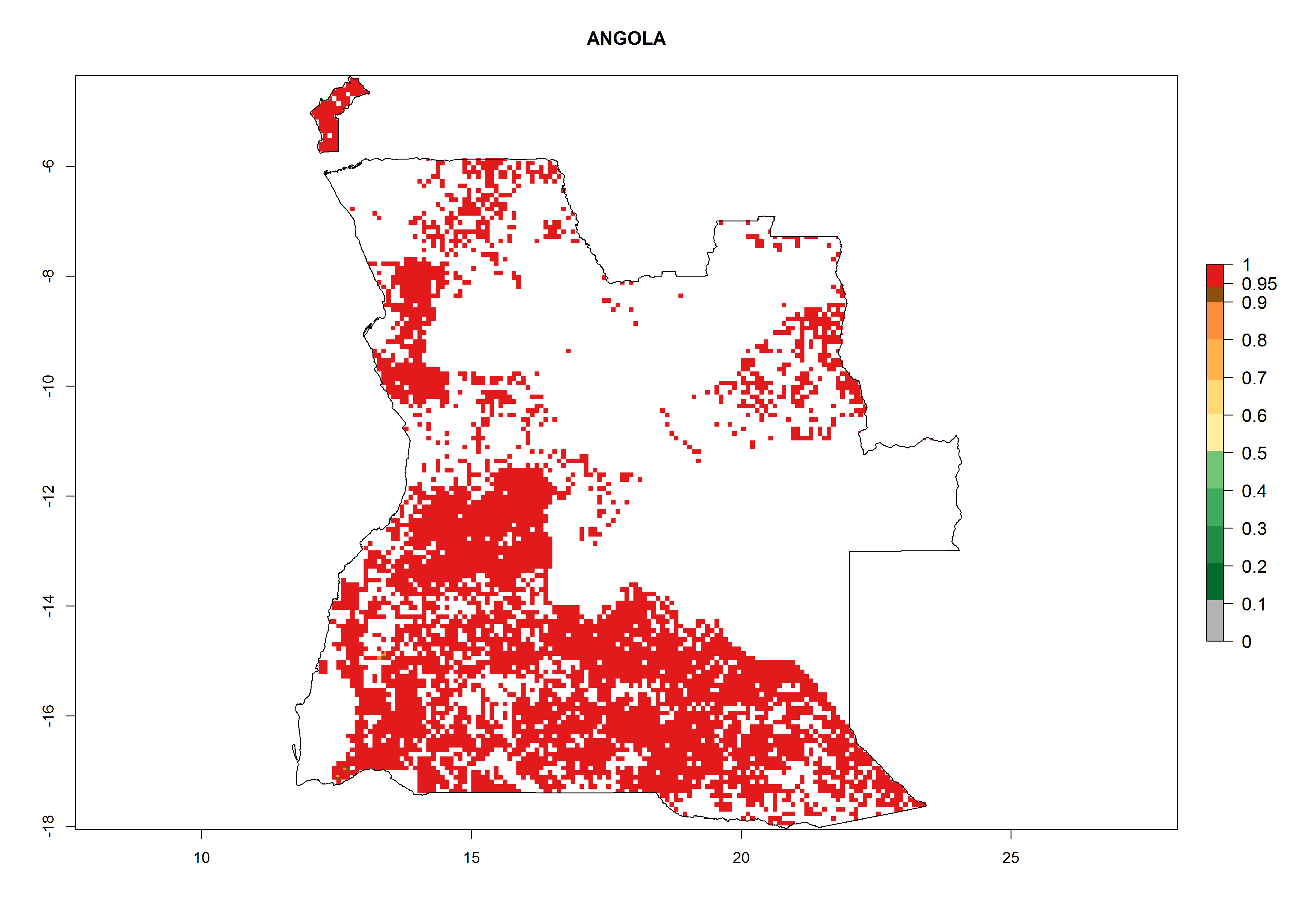
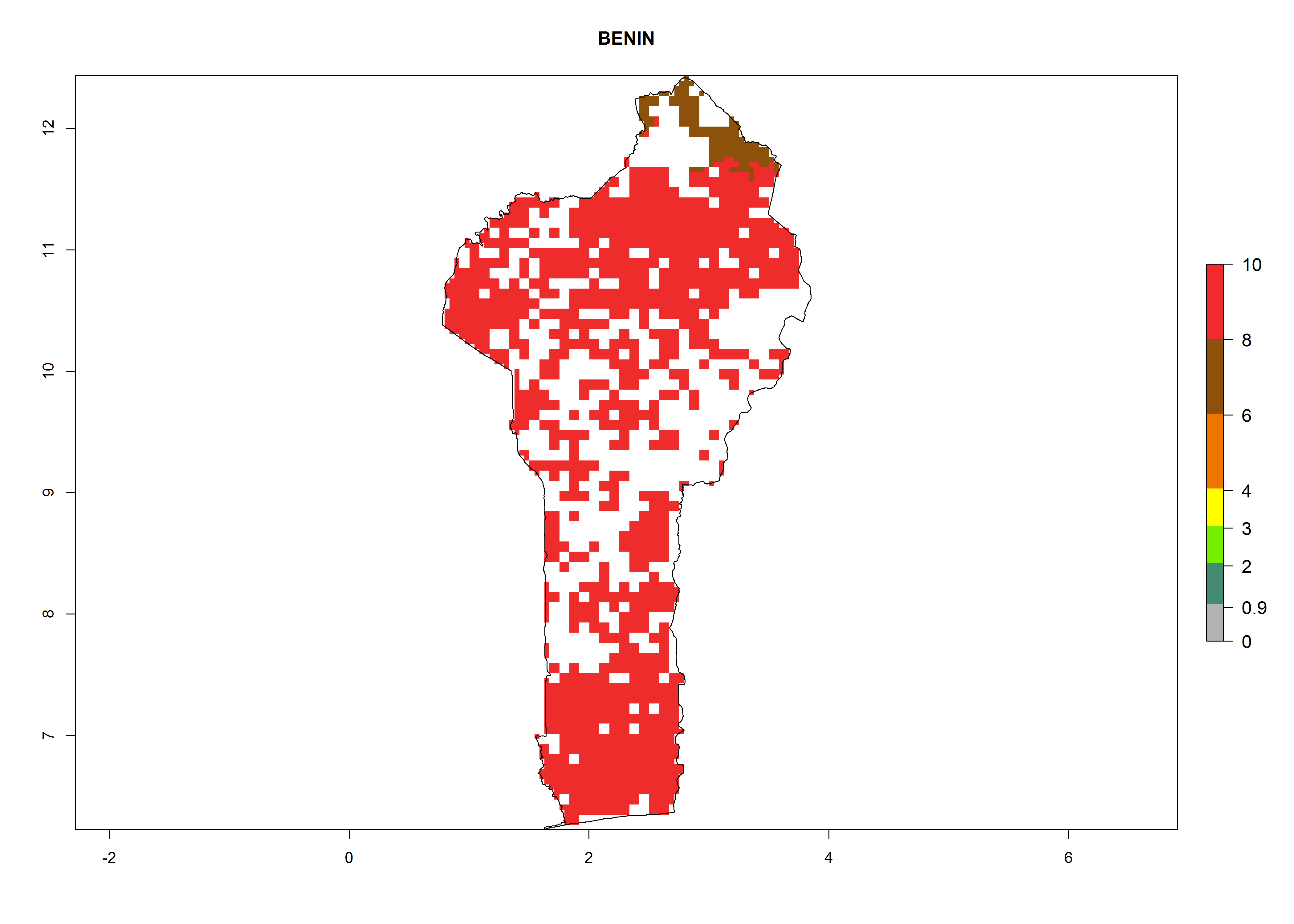
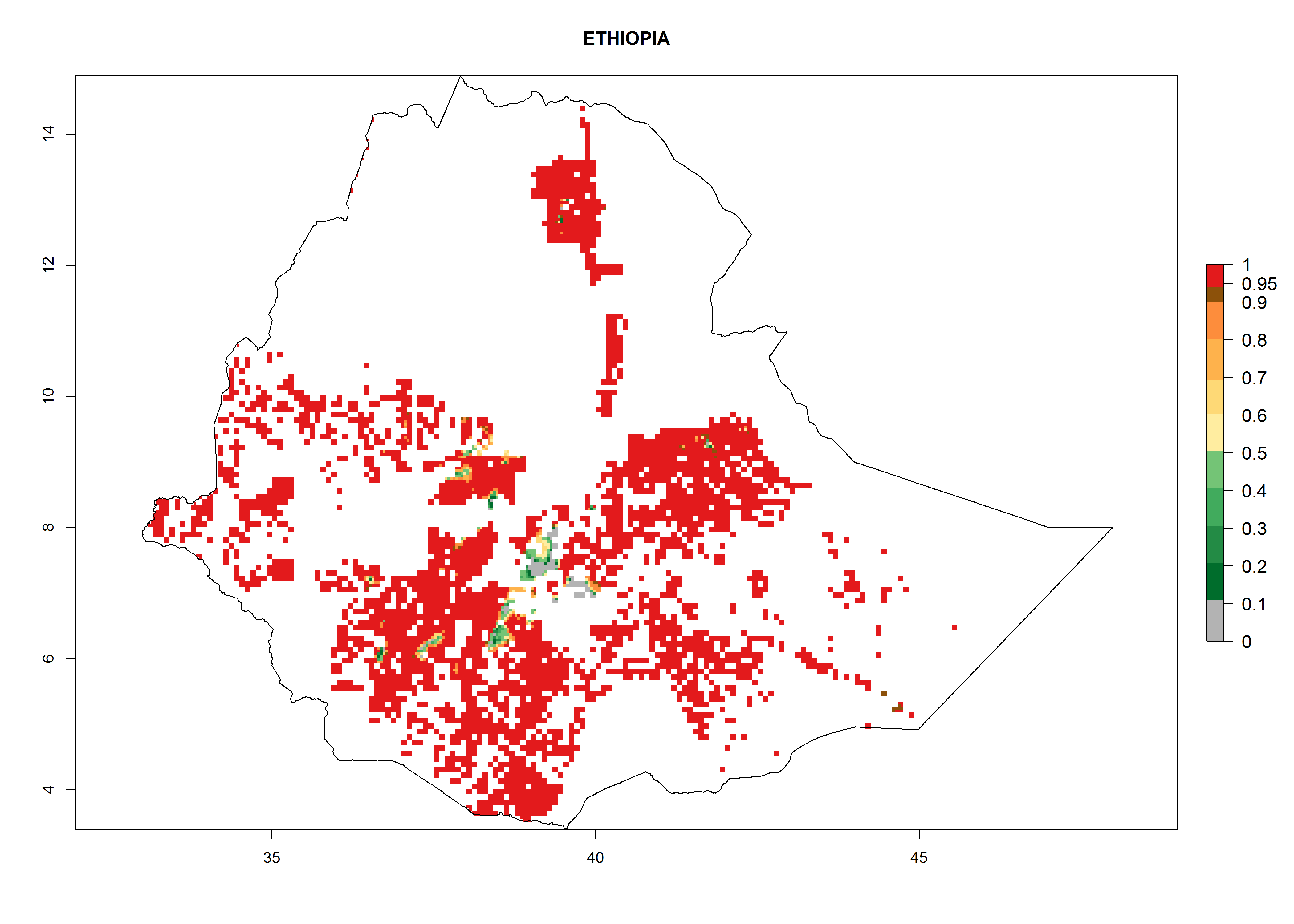
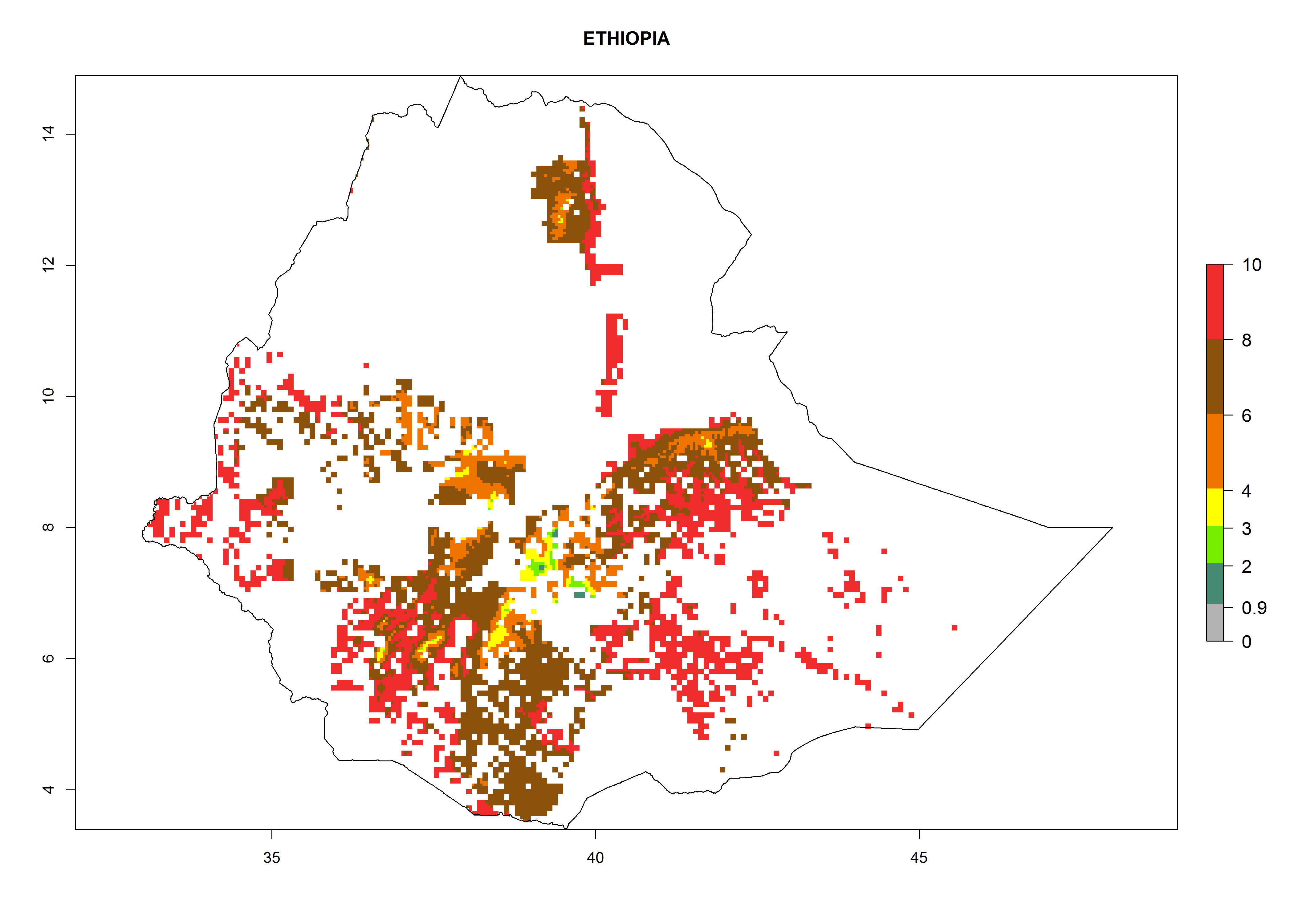
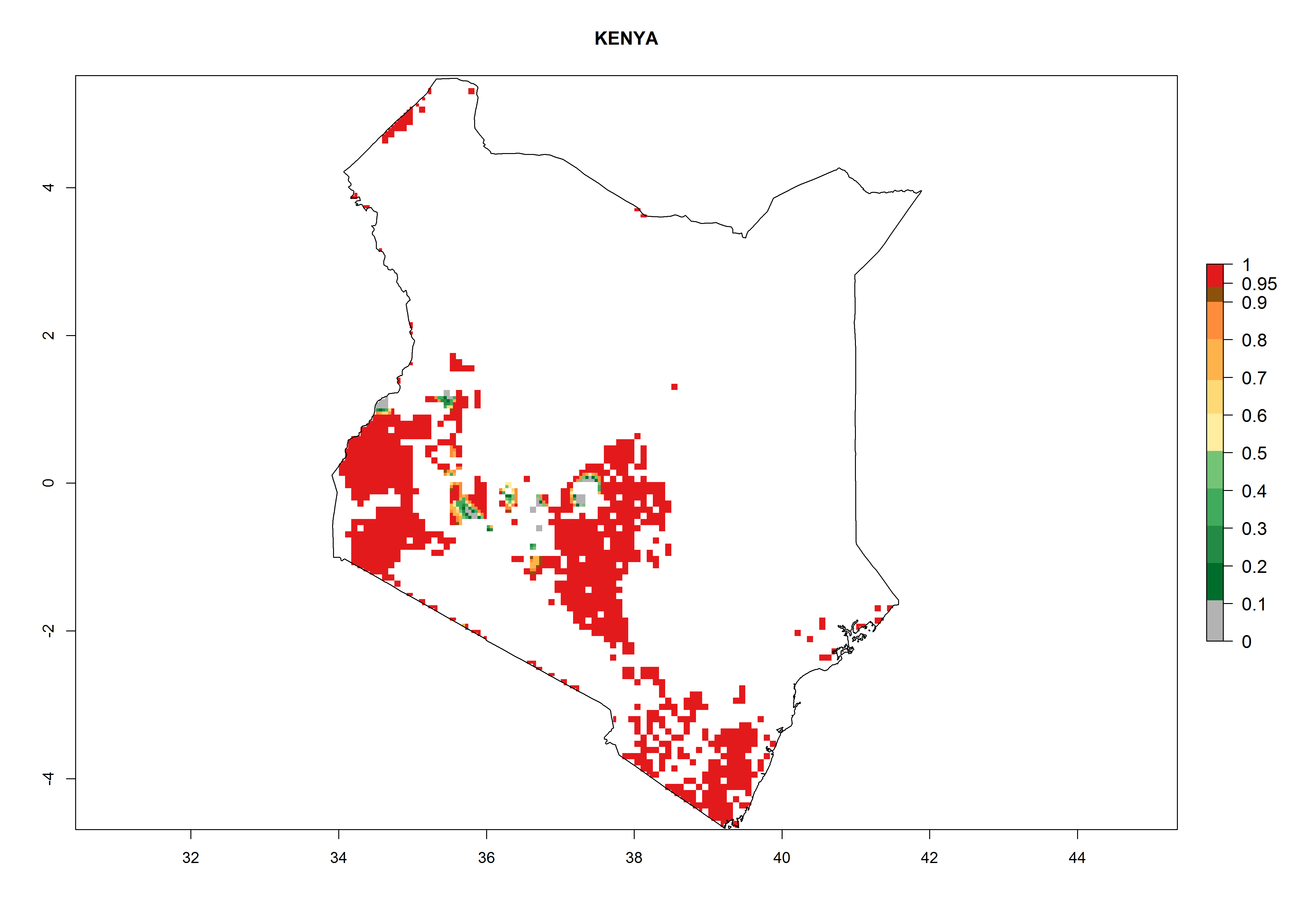
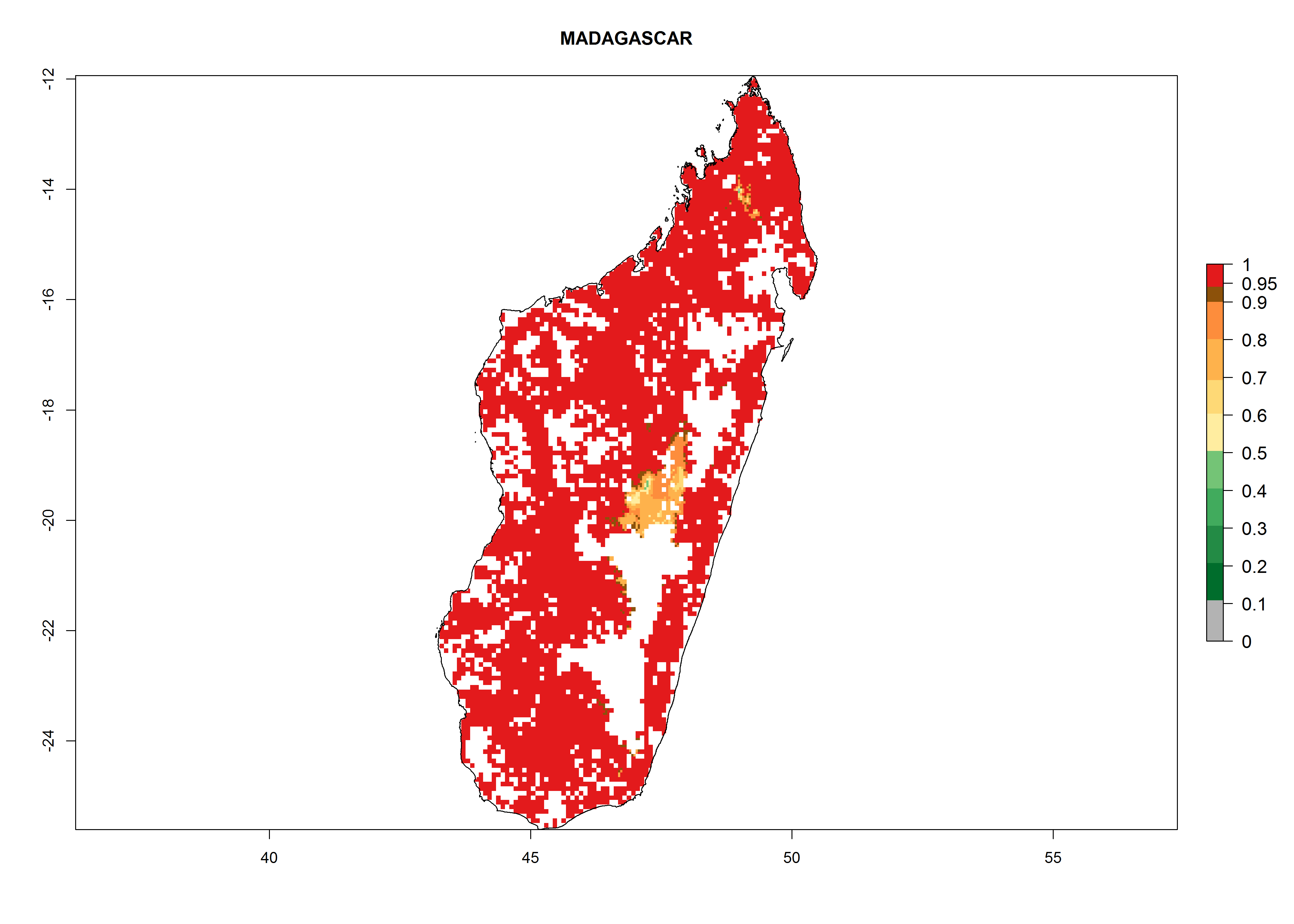
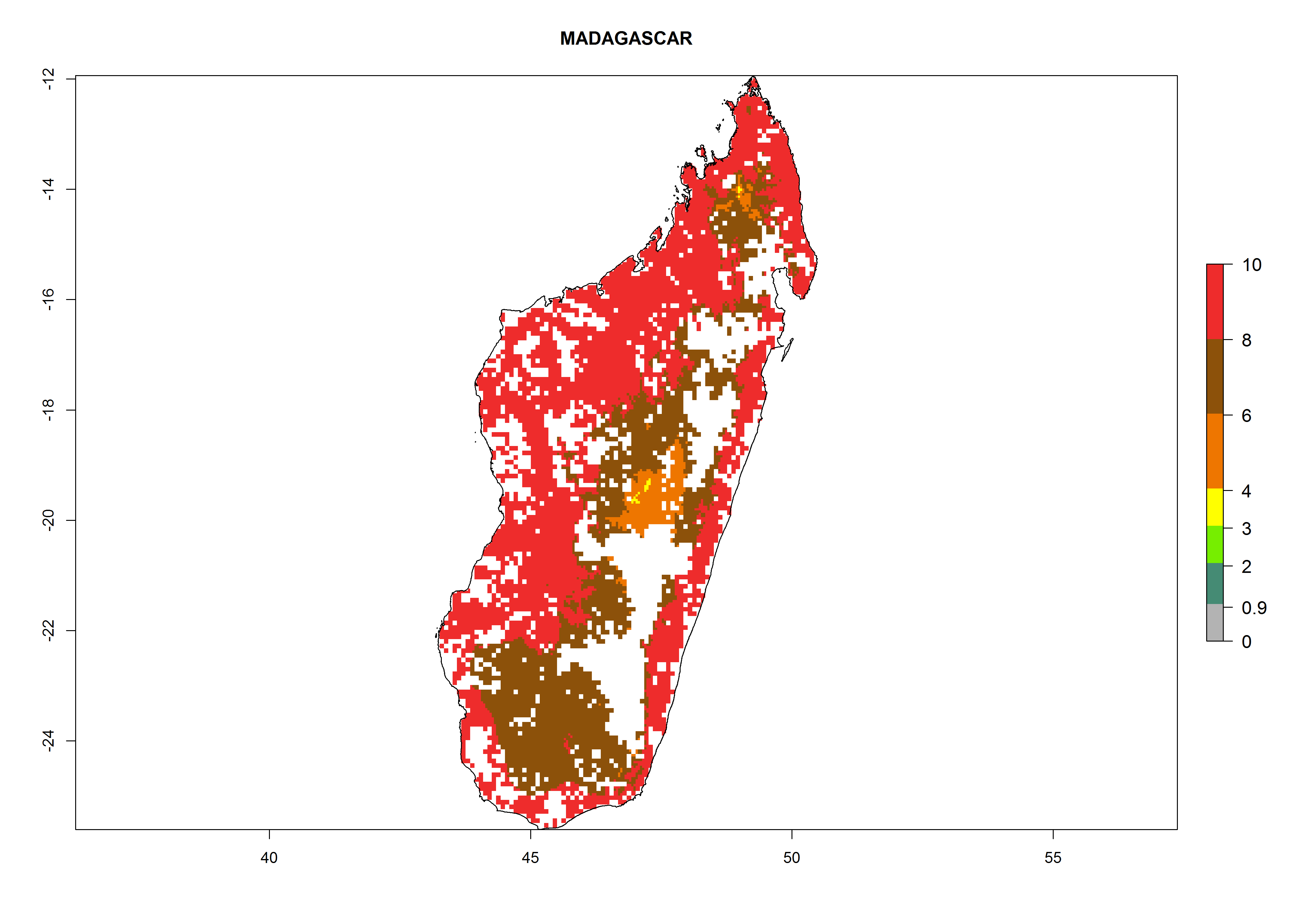
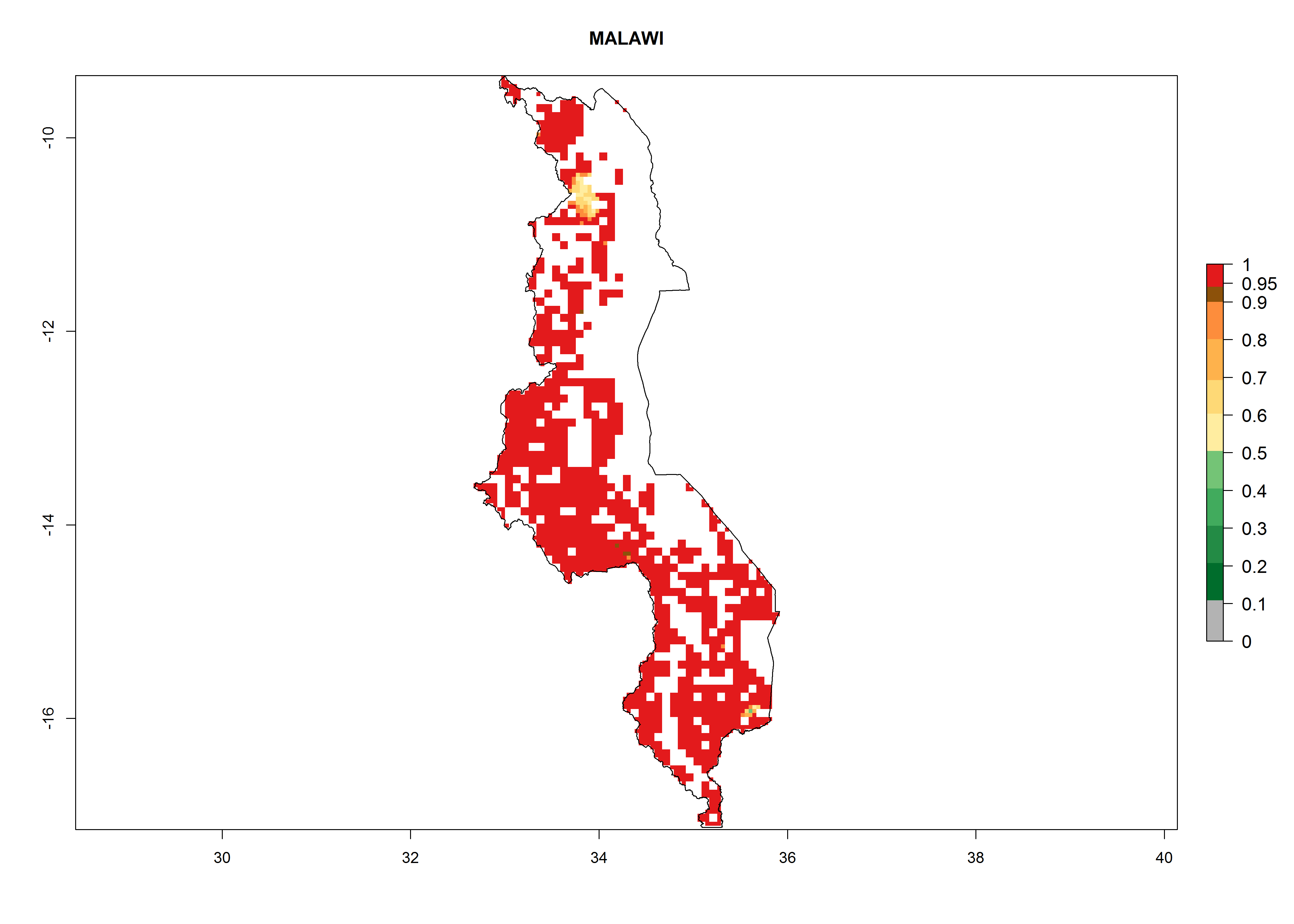
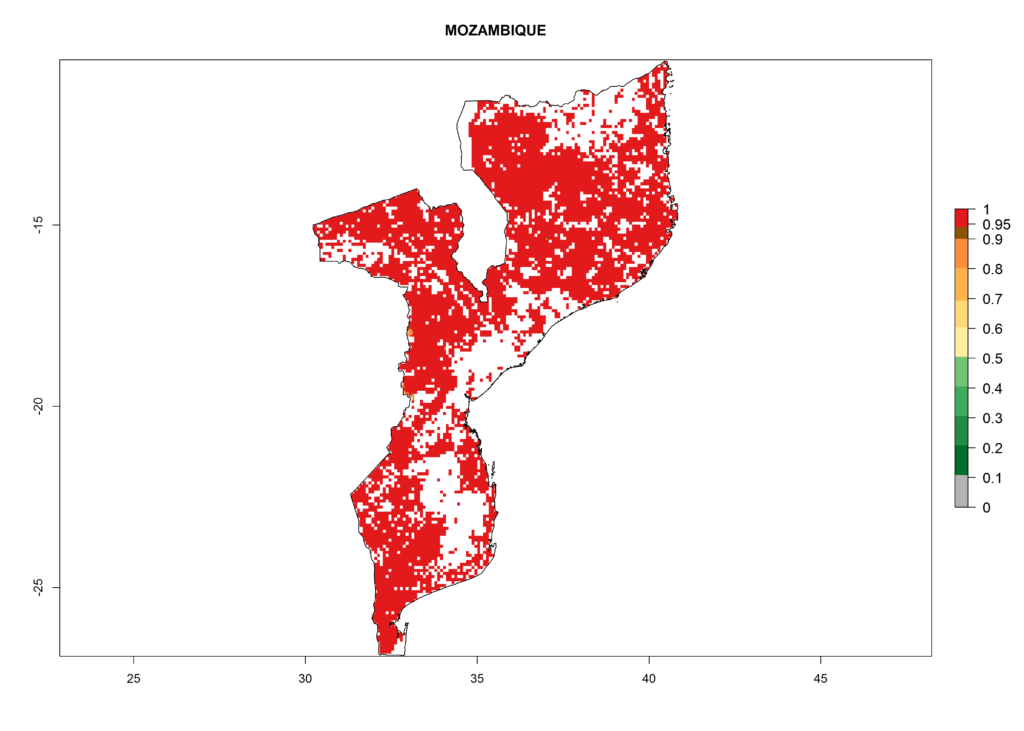
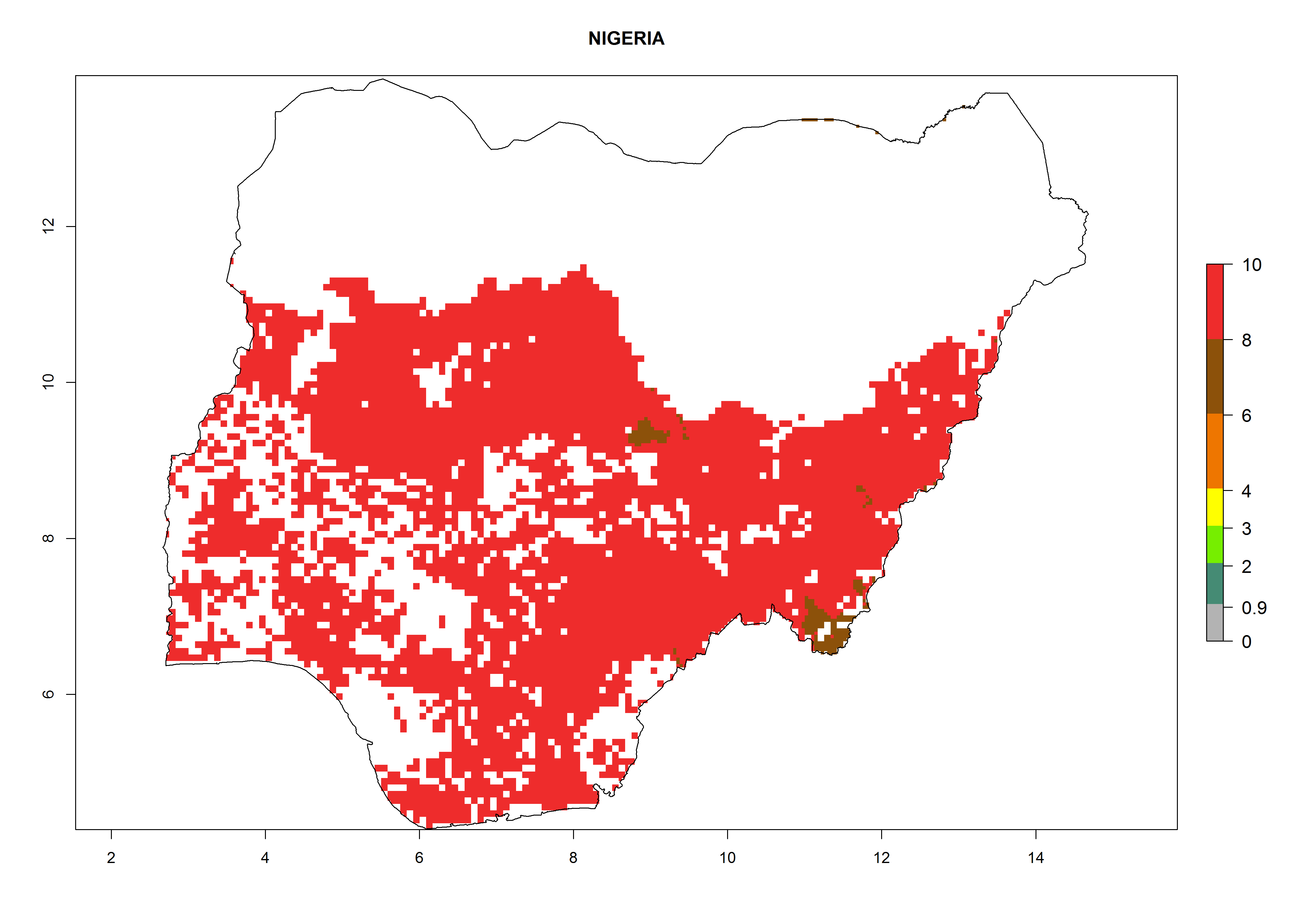
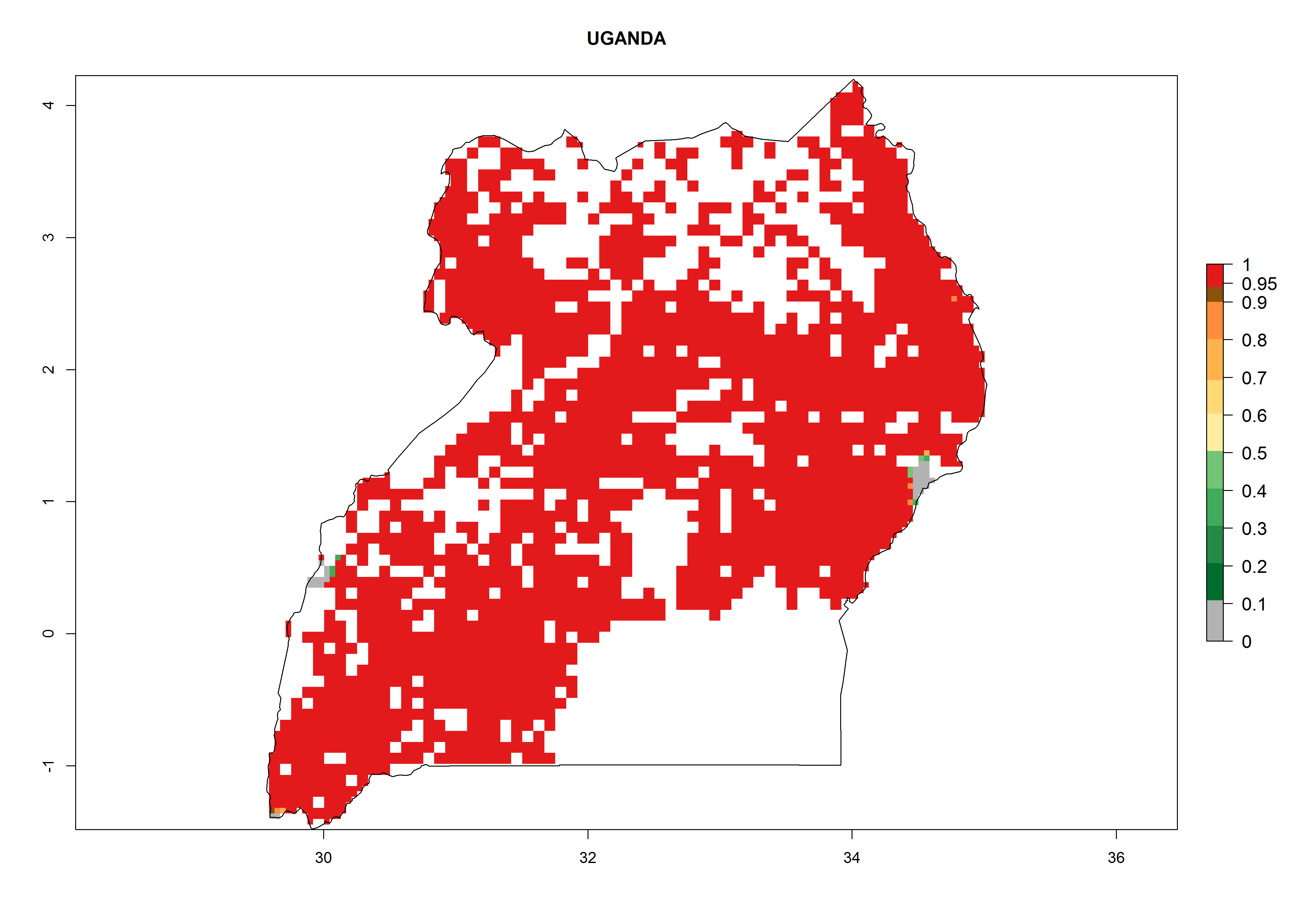
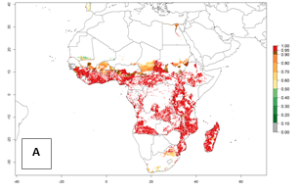
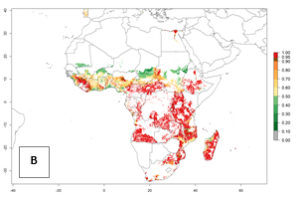
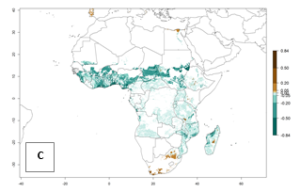
In Africa, B. afer is already present in West Africa (Benin, Burkina Faso, Ghana, Ivory Coast, Nigeria, Sierra Leone); Central Africa (Angola); East Africa (Uganda, Ethiopia, Kenya, Tanzania, Madagascar, Malawi, Mozambique); and North Africa (Sudan), with a predicted ERI>0.95, as well as in South Africa and Chad, with an ERI>0.6–0.7 (Fig. 5A). Under the year 2050 temperature scenario, the risks of establishment will potentially decrease in countries south of the Sahel in West and Central Africa, but B. afer will remain or increase its potential threat and risk in countries of Southern and East Africa (Fig. 5B, C). These areas are important for the production of food crops such as sweetpotato that are affected by viral diseases transmitted by this species.
 |
 |
 |
|
Figure 5. Changes in establishment and potential distribution of the whitefly, Bemisia afer, in African sweetpotato production regions according to model predictions, using the ERI for the years 2000 (A) and 2050 (B), and changes of the ERI between 2000 and 2050 (C). An ERI>0.95 is associated with potential permanent establishment.
Changes in abundance
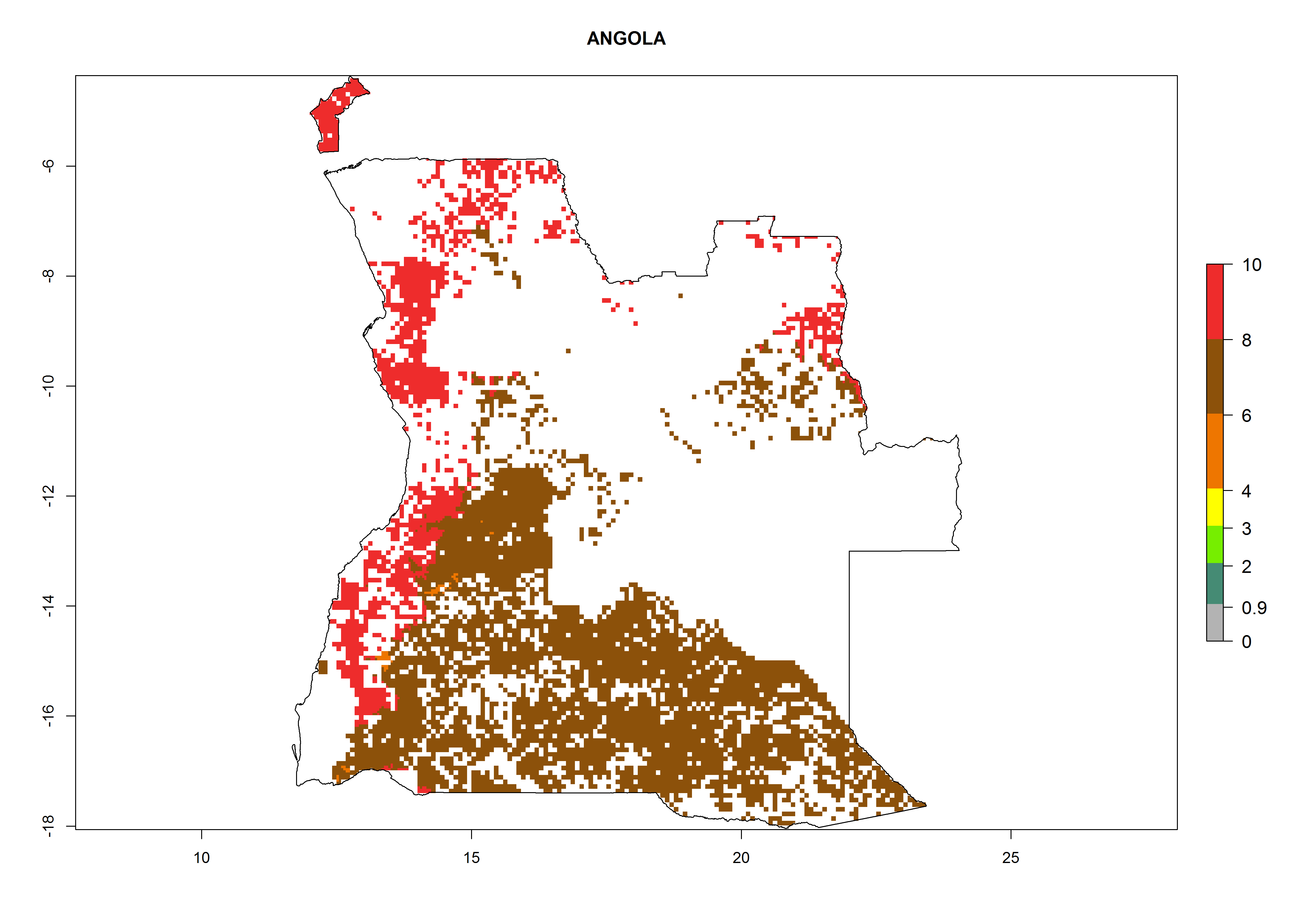
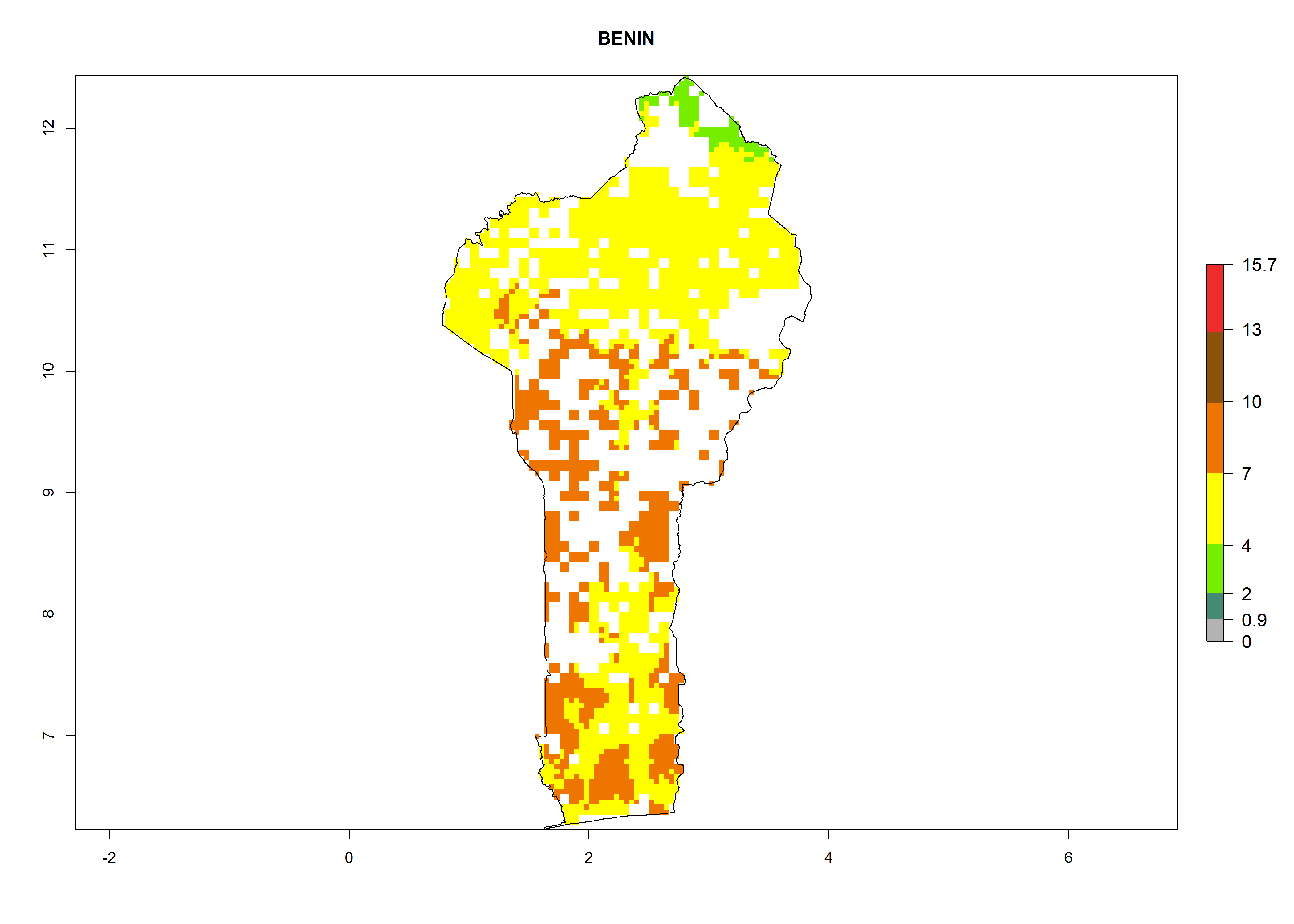
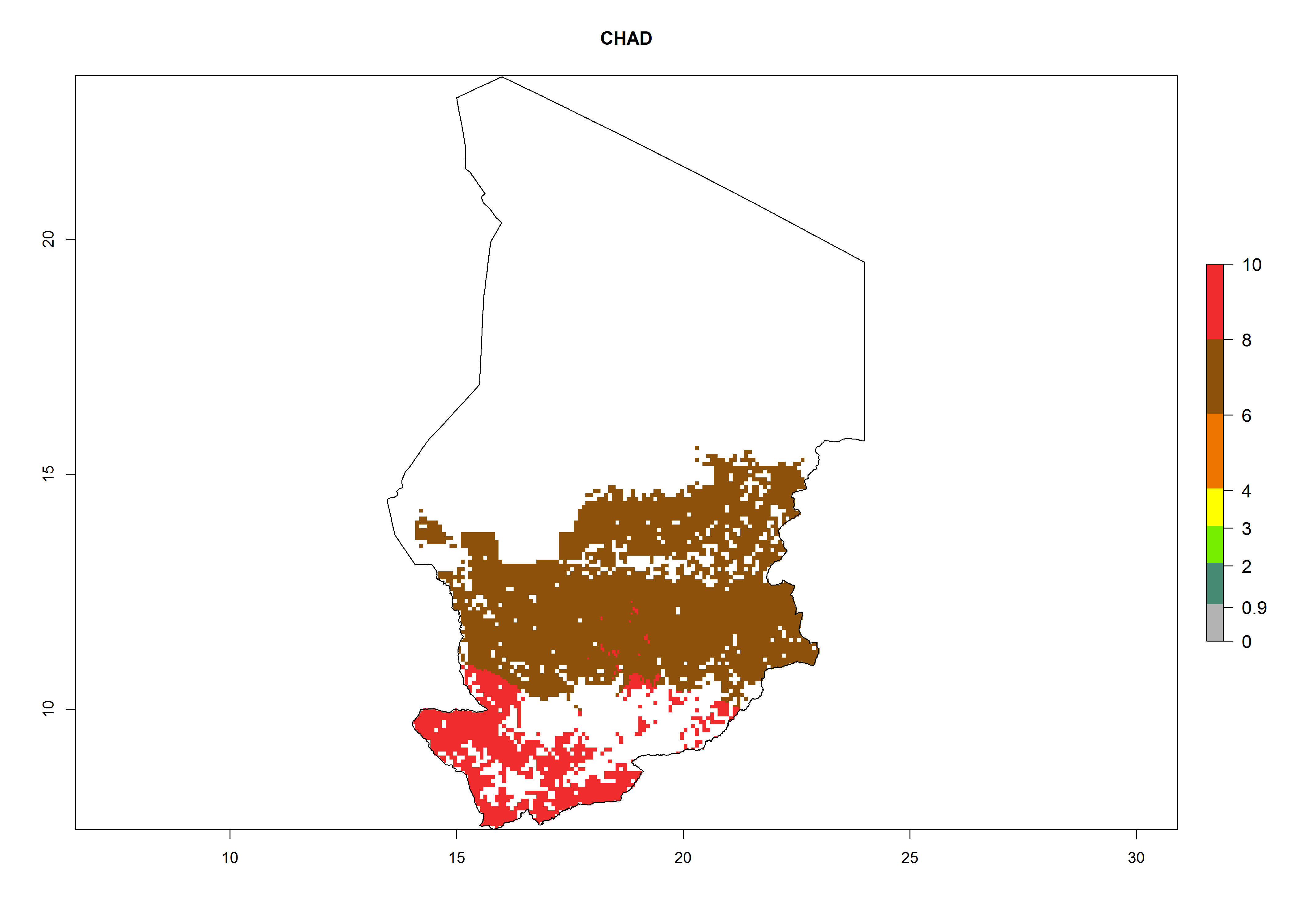
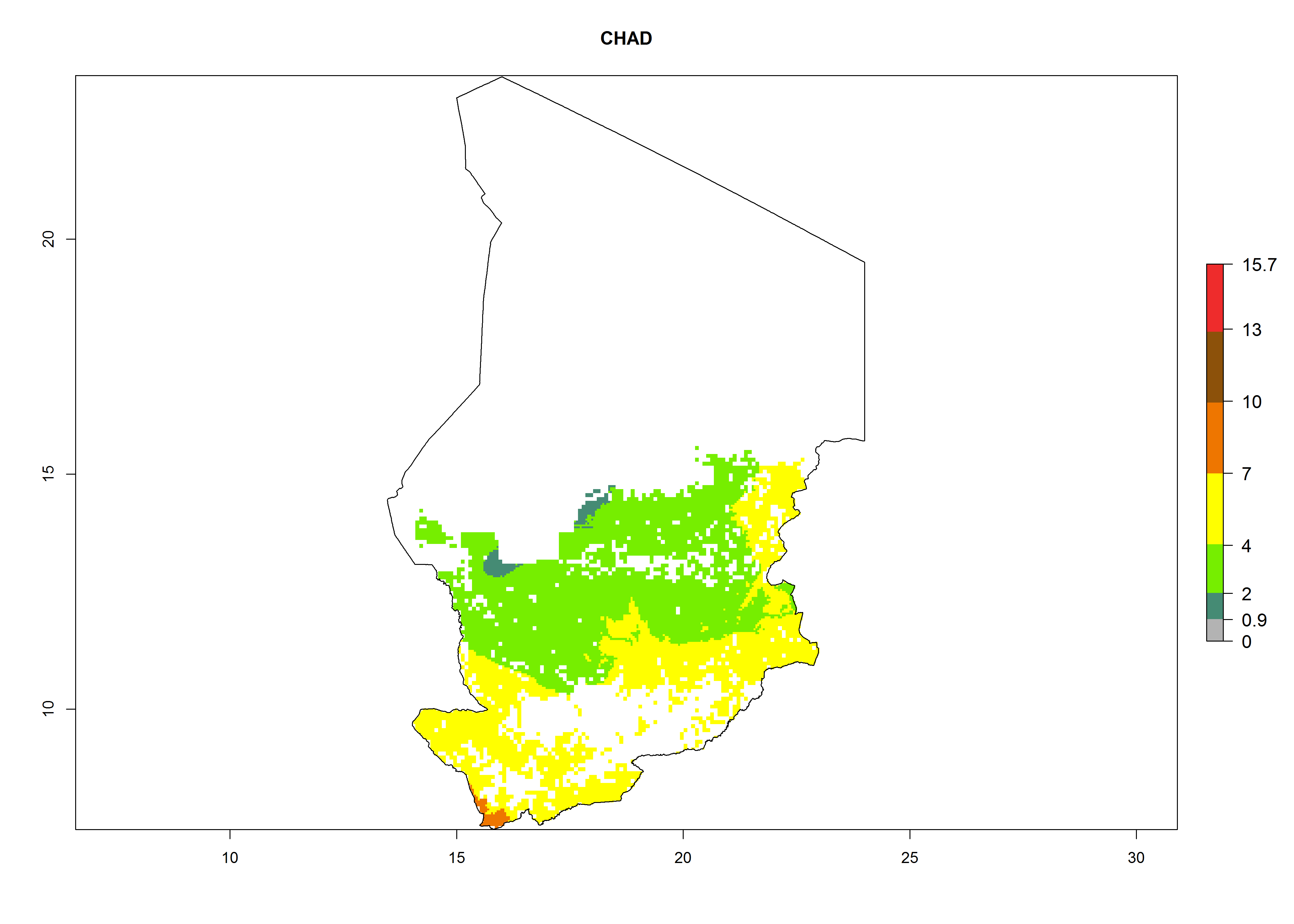
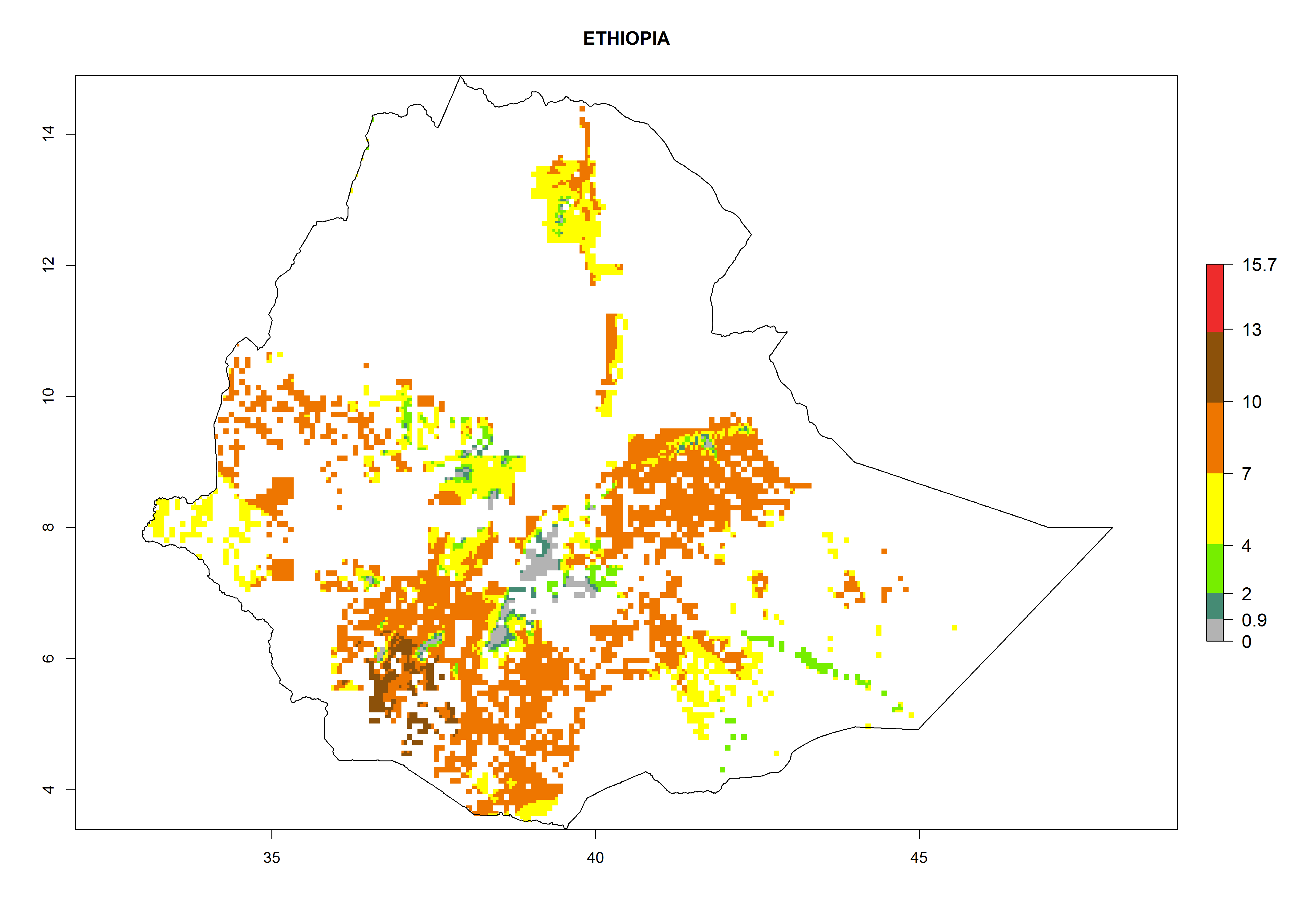
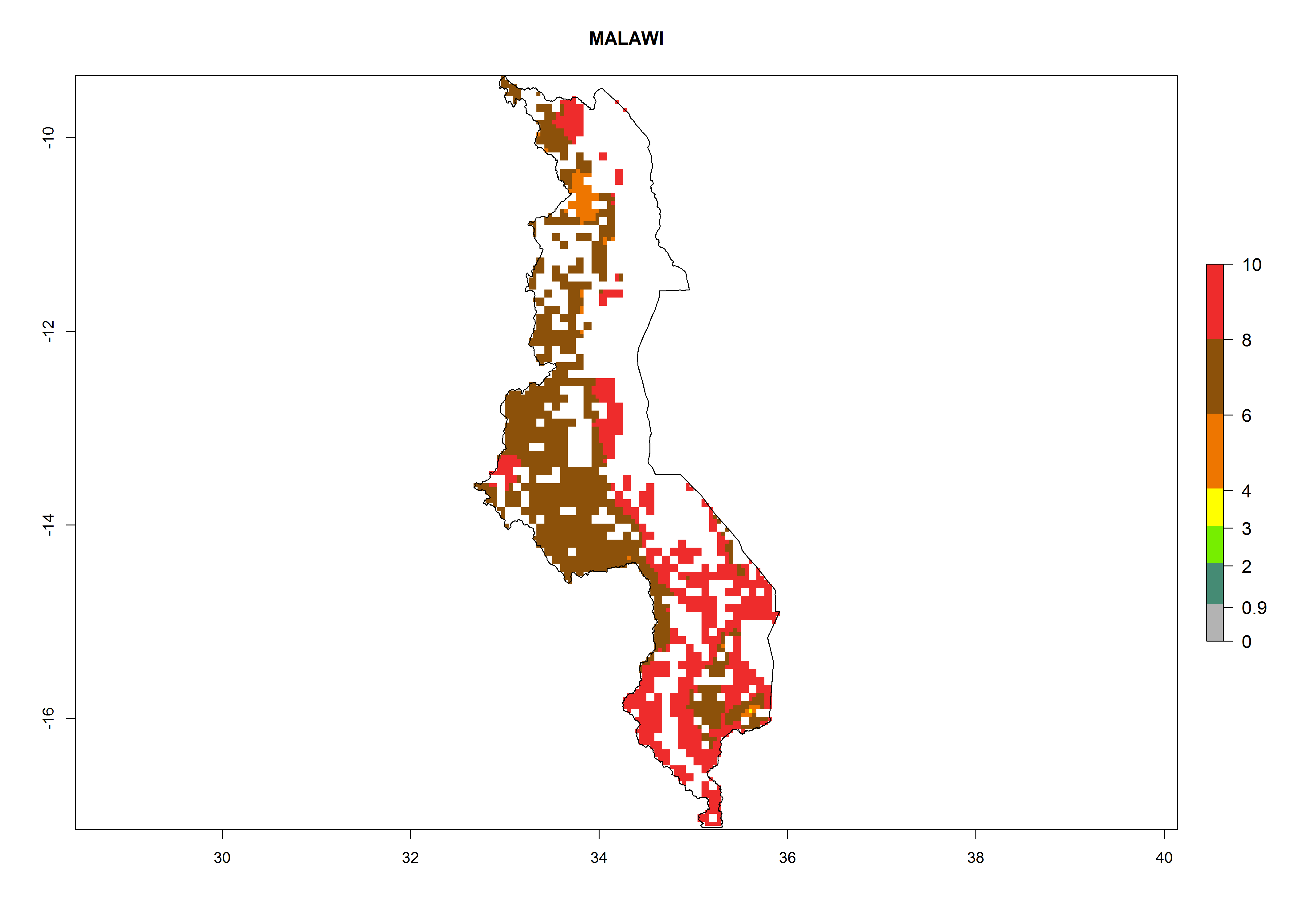
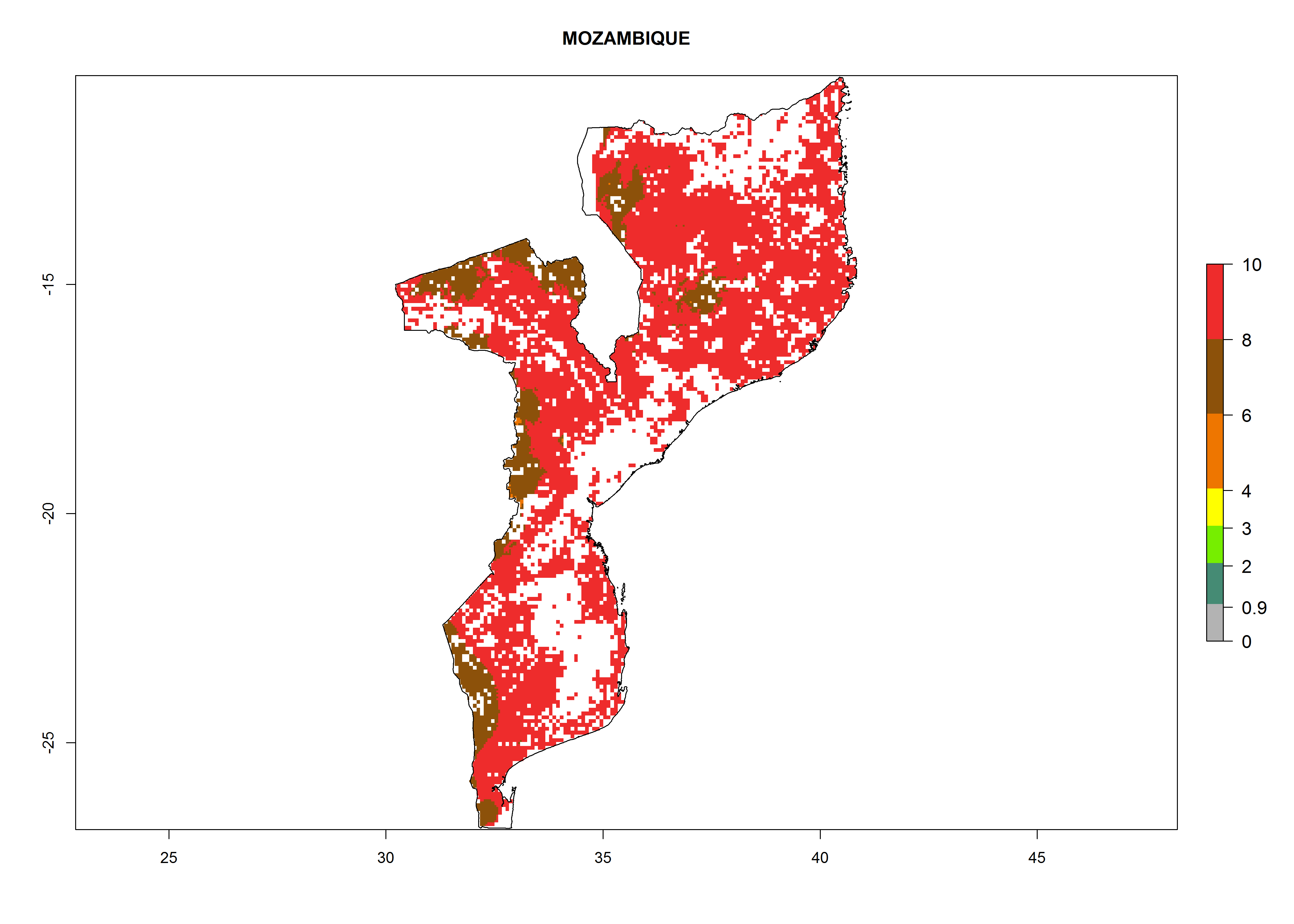
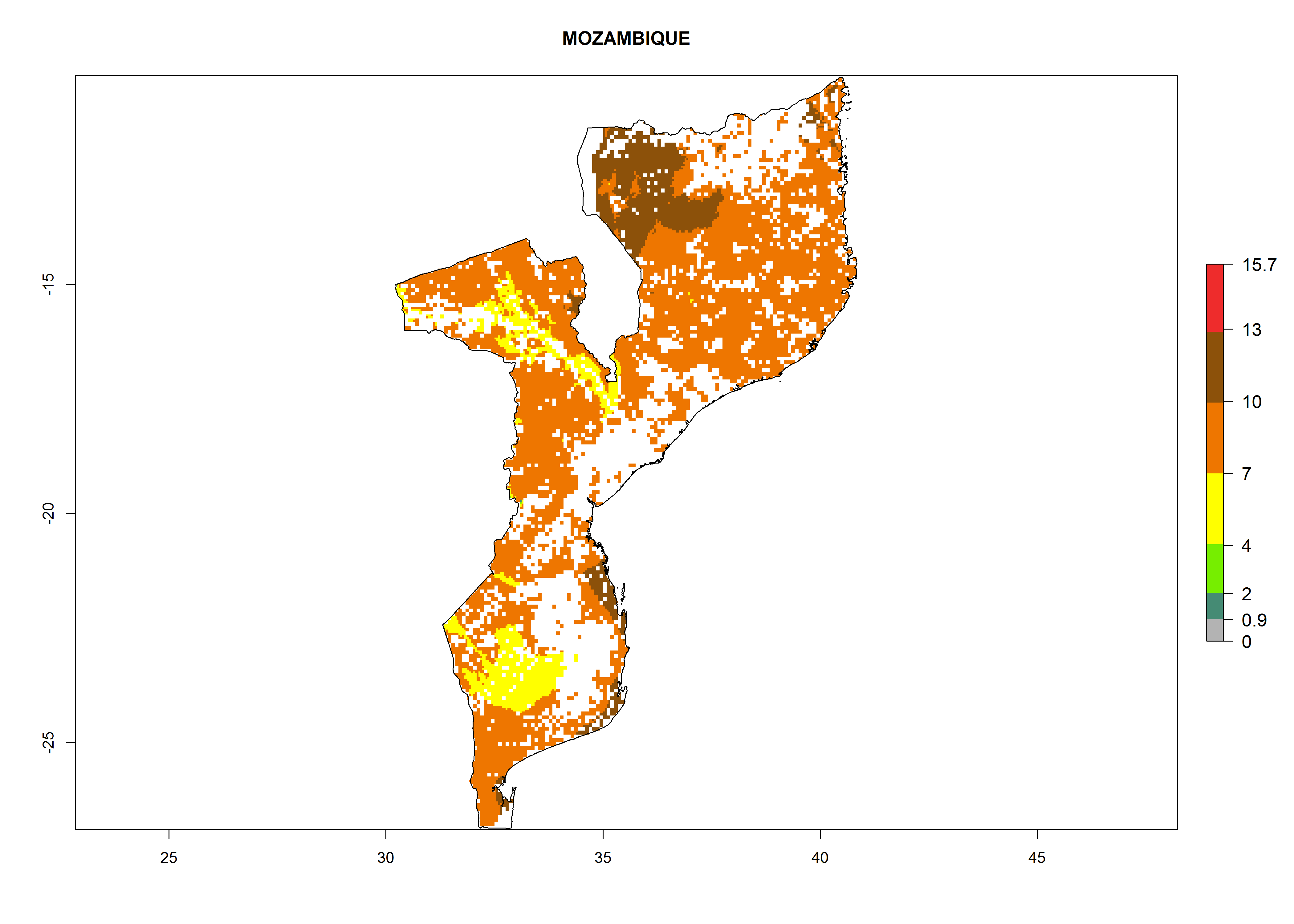
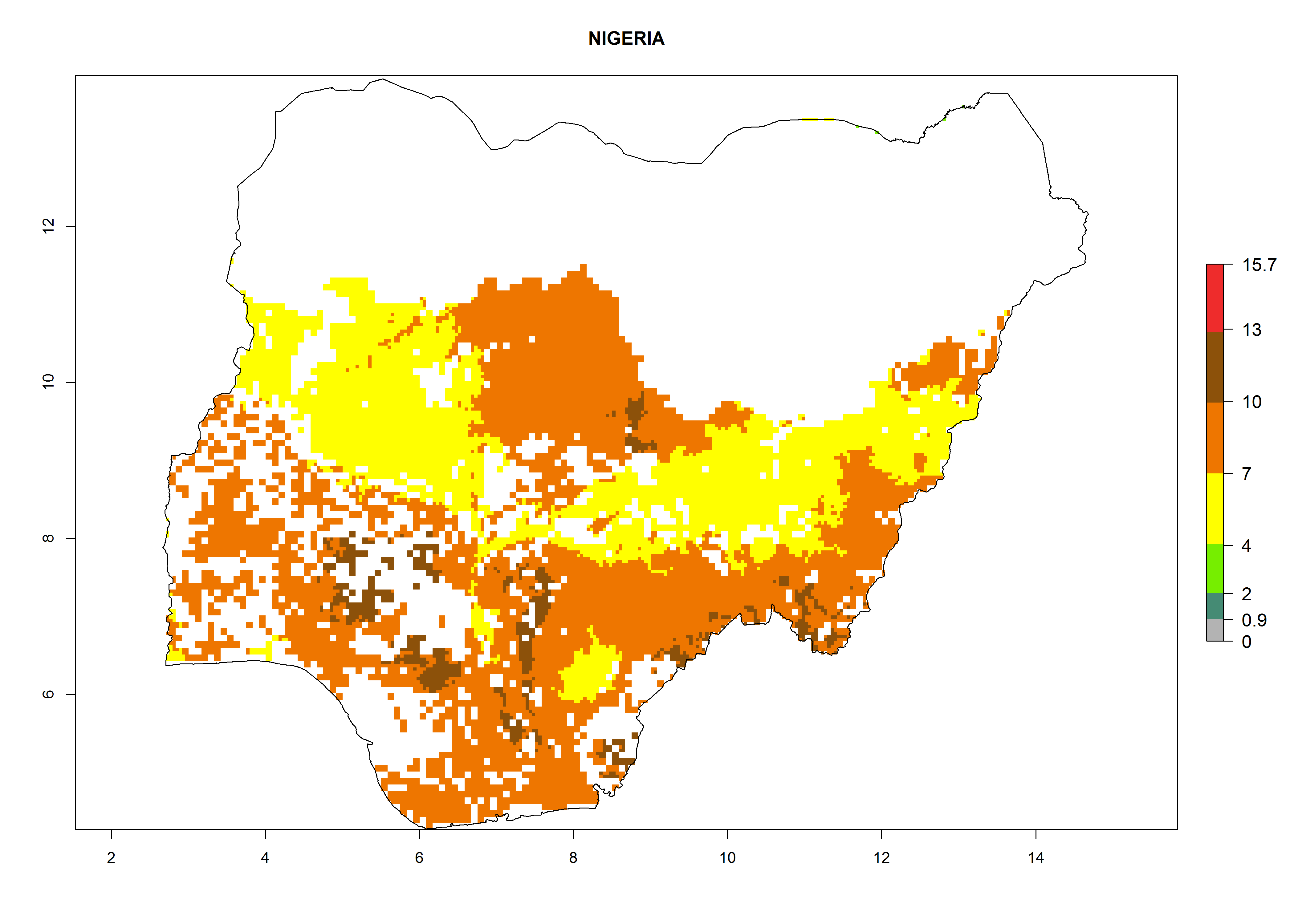
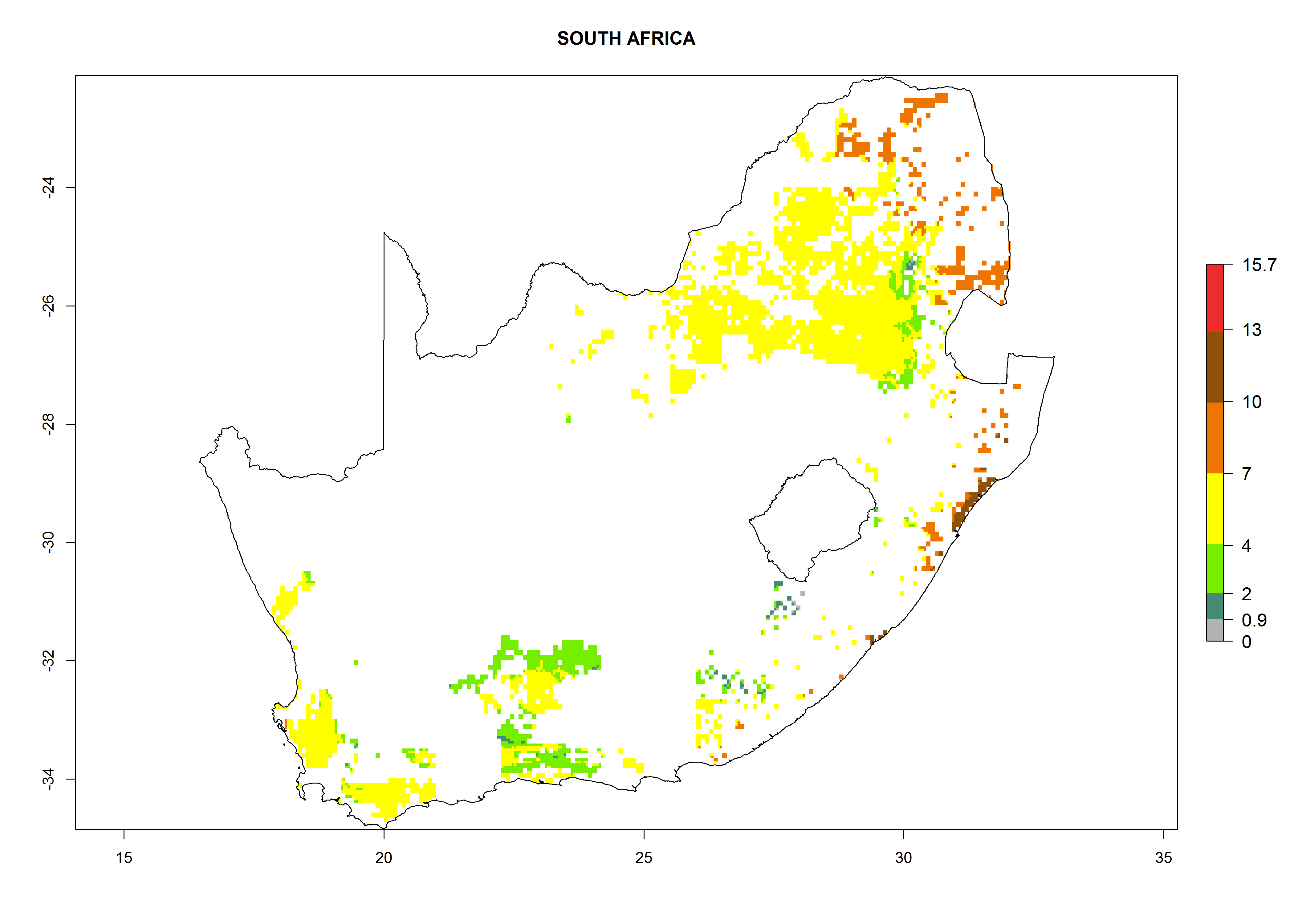
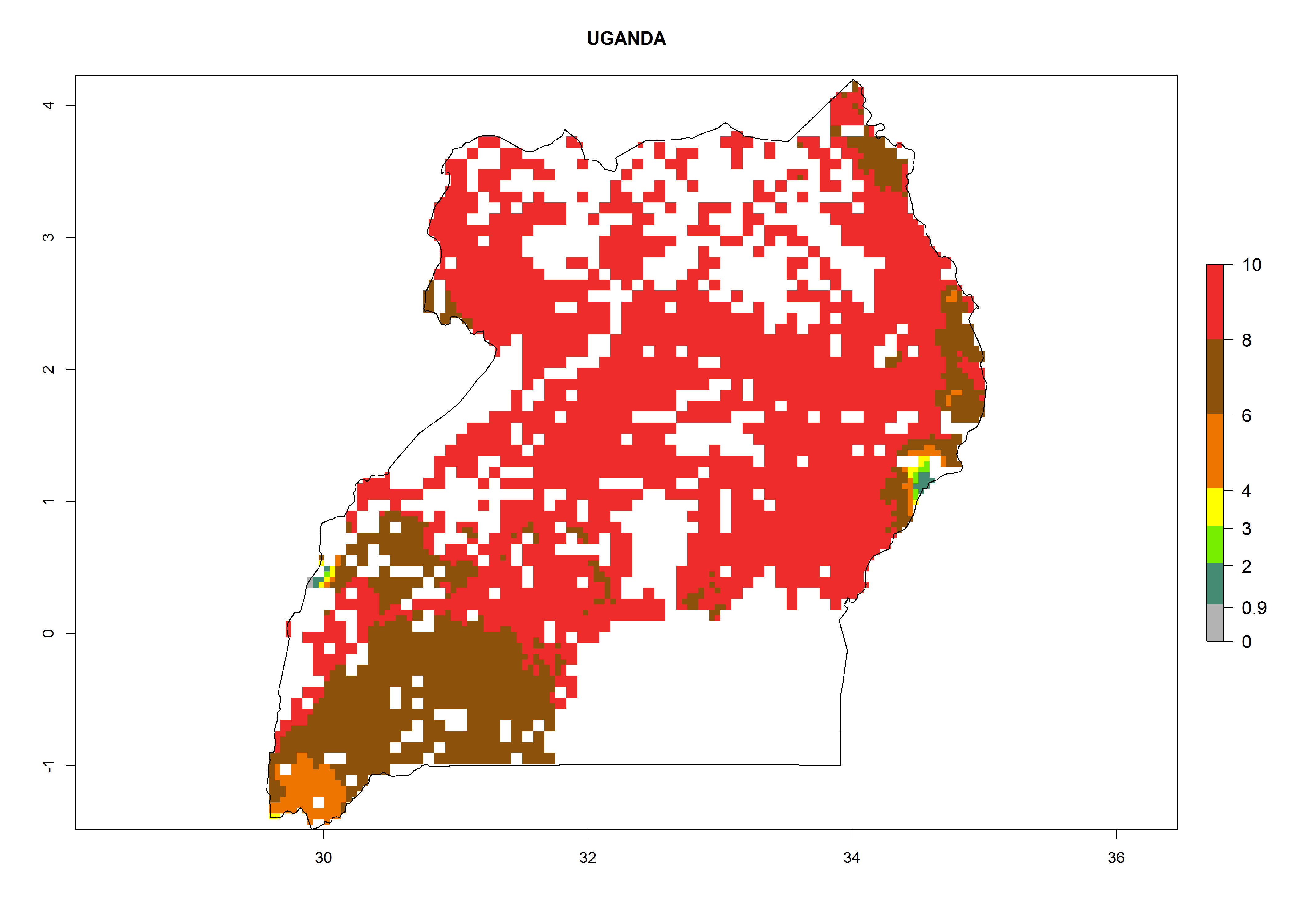
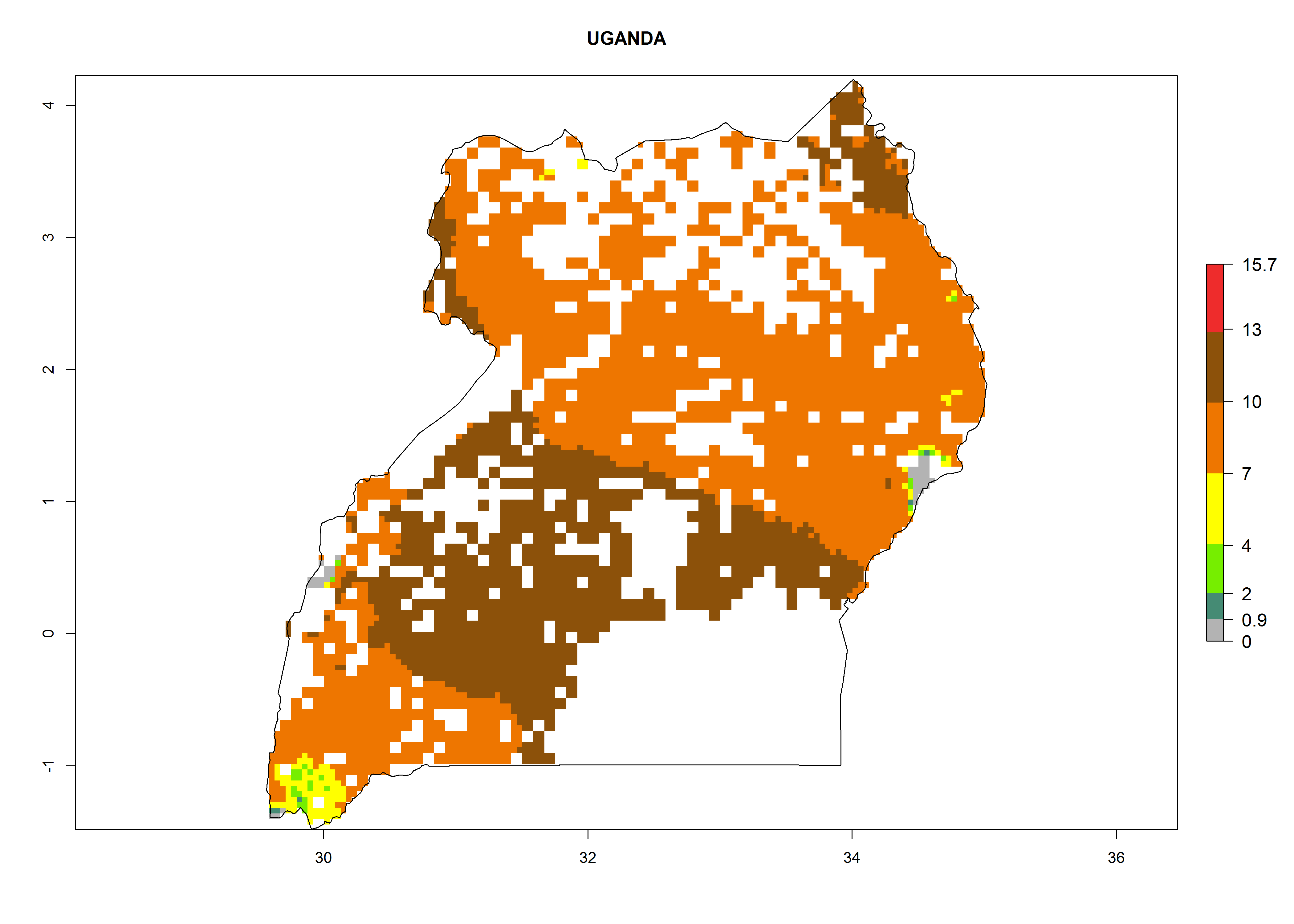
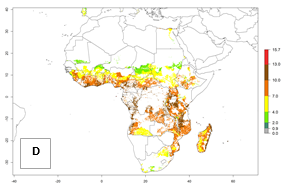
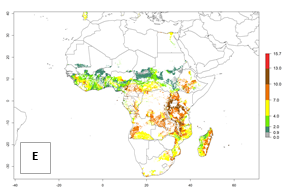
In Africa, the highest increase in the number of B. afer generations (1–3 per year) is projected for most southeastern countries (Fig. 6C). It is estimated that under the year 2050 temperature scenario, the number of generations will increase (Fig. 6B, C). Further, an increase in the population growth potential (AI>1–5) is projected for regions in Southern and East Africa (Ethiopia, Kenya, Uganda, Tanzania, Malawi, South Africa, Madagascar) and an increase in the population growth potential of an AI>1–2 in Angola (Fig. 6F).
| GI | AI | |
| 2000 | 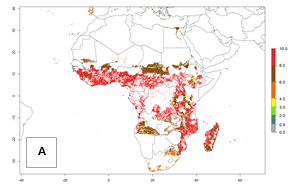 |
 |
| 2050 | 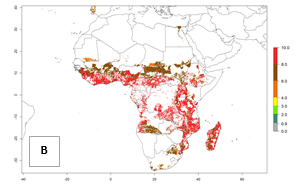 |
 |
| Index change (2000 – 2050) | 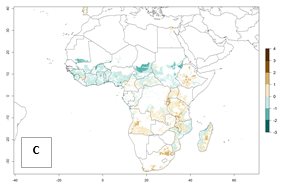 |
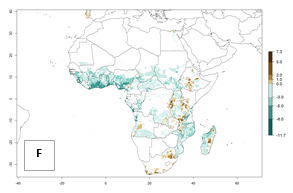 |
Figure 6. Changes in abundance (GI, damage potential) and activity (AI, potential population growth) of the whitefly, Bemisia afer, in African sweetpotato production regions according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
Country Risk Maps
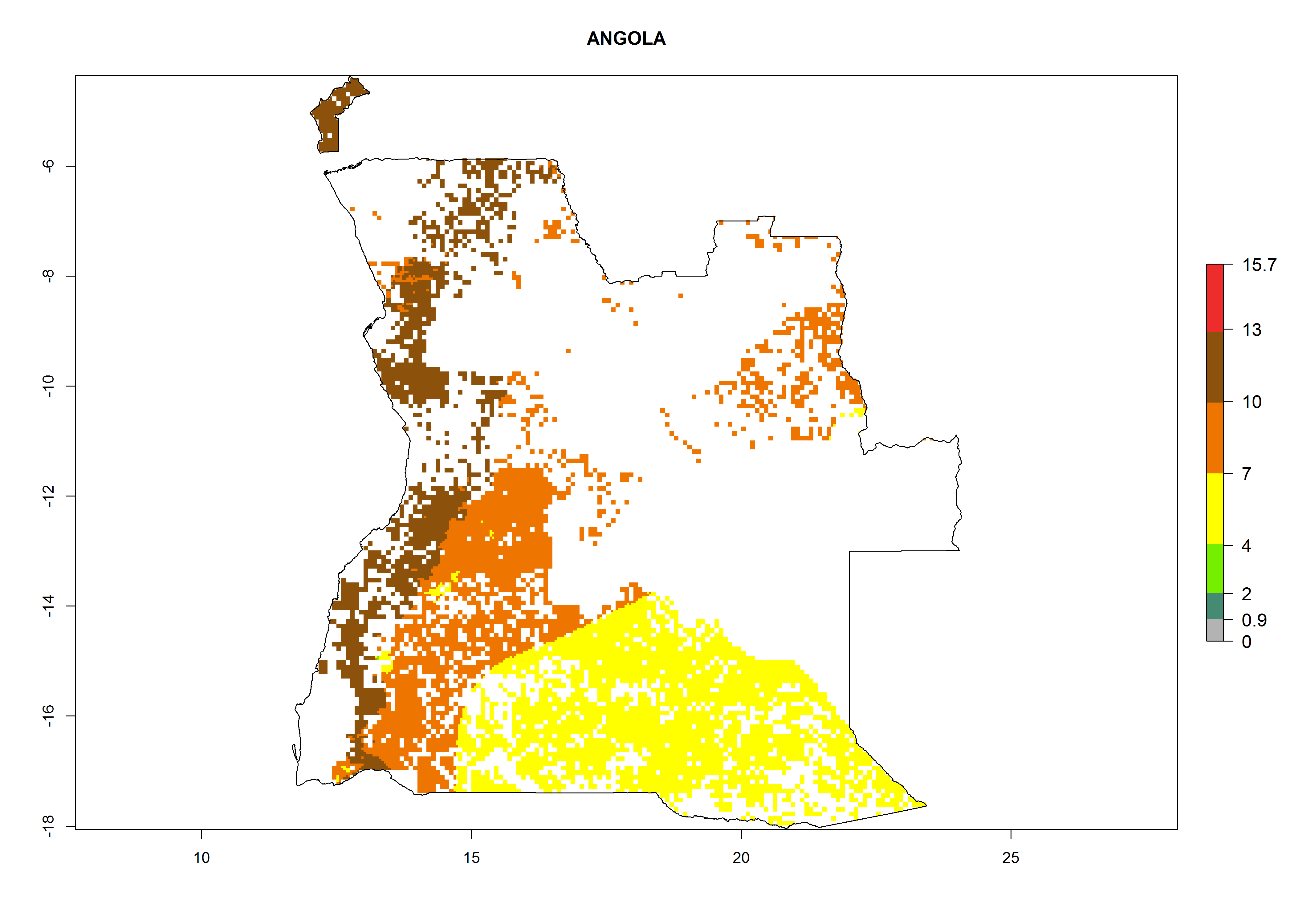
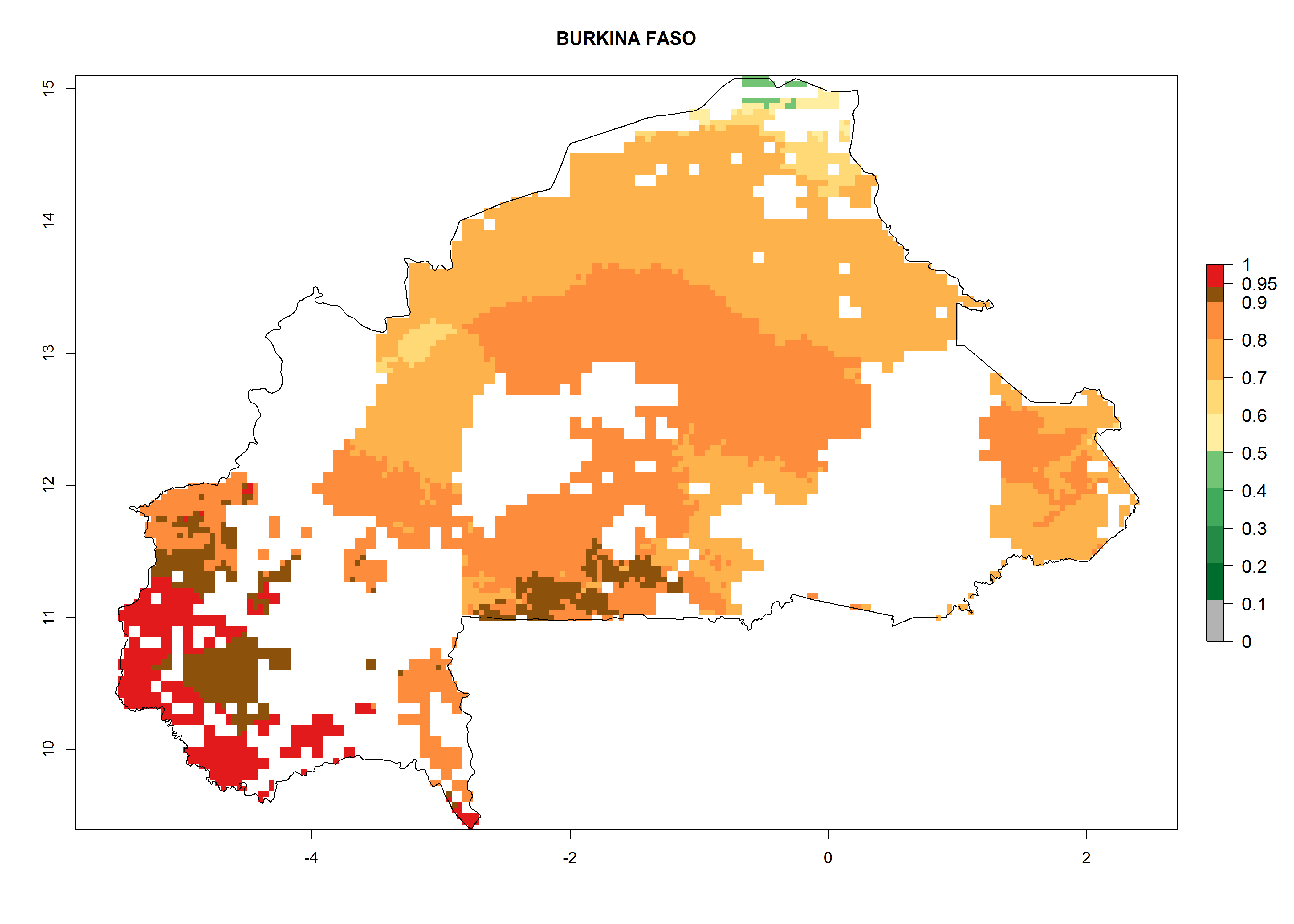
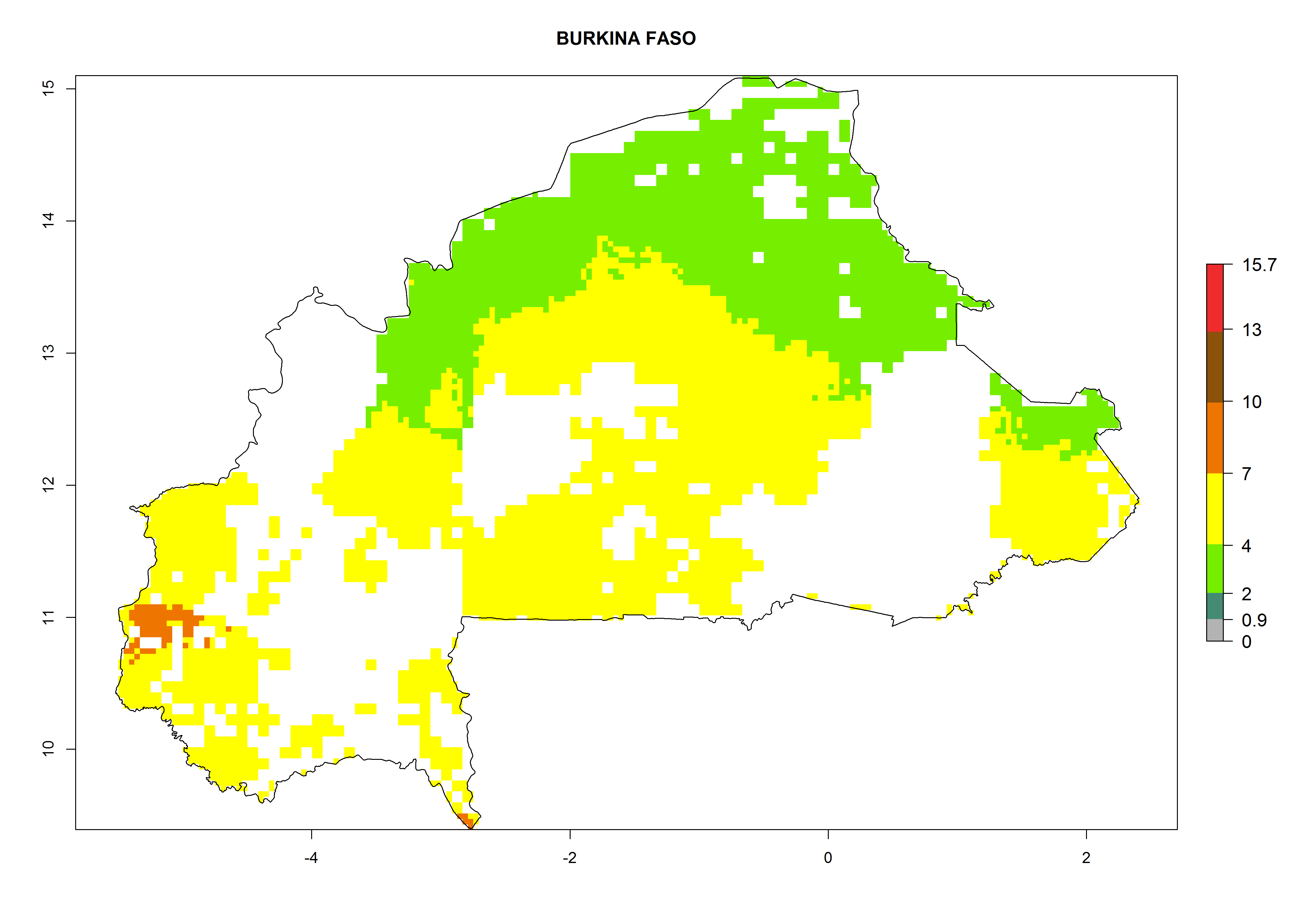
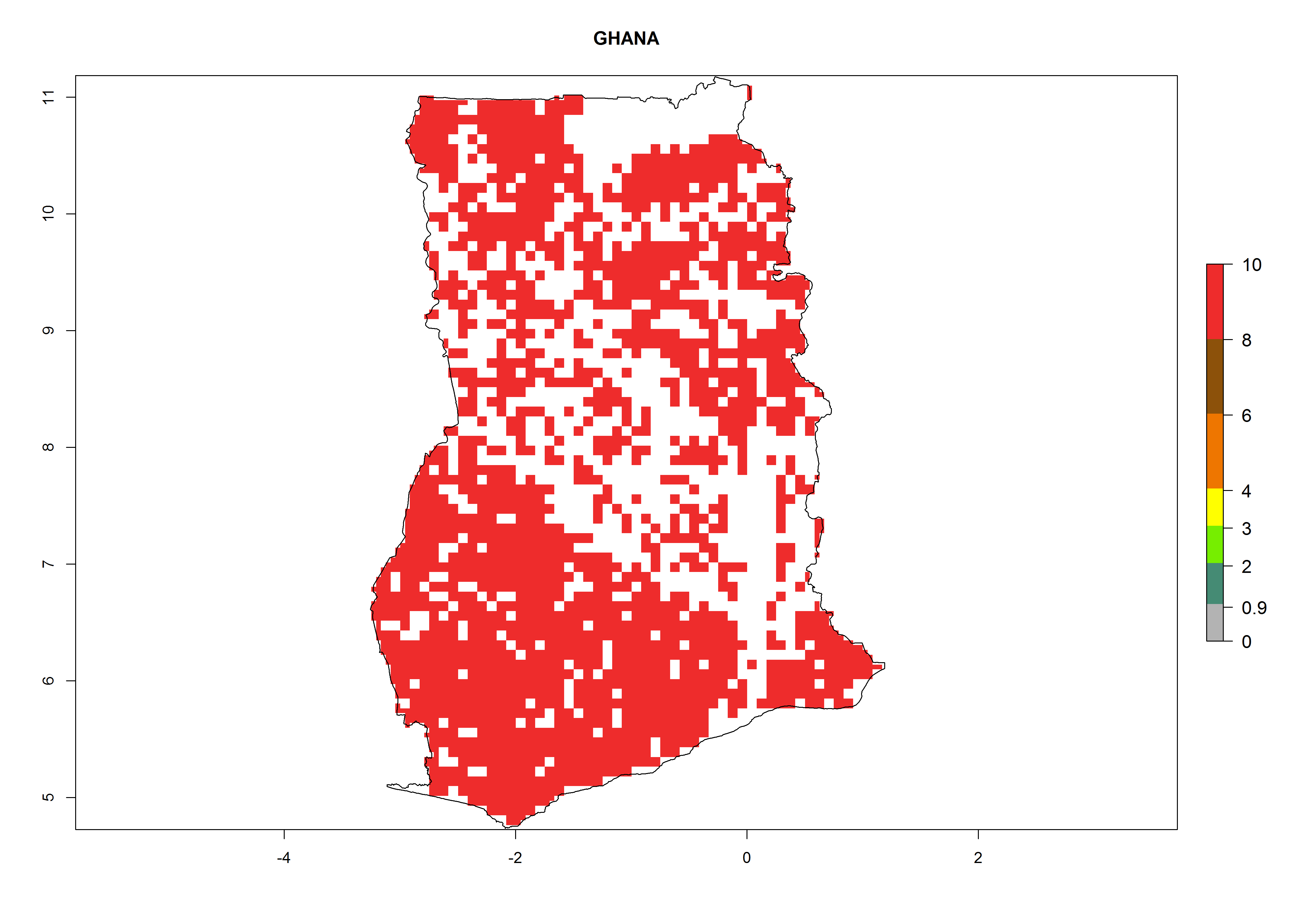
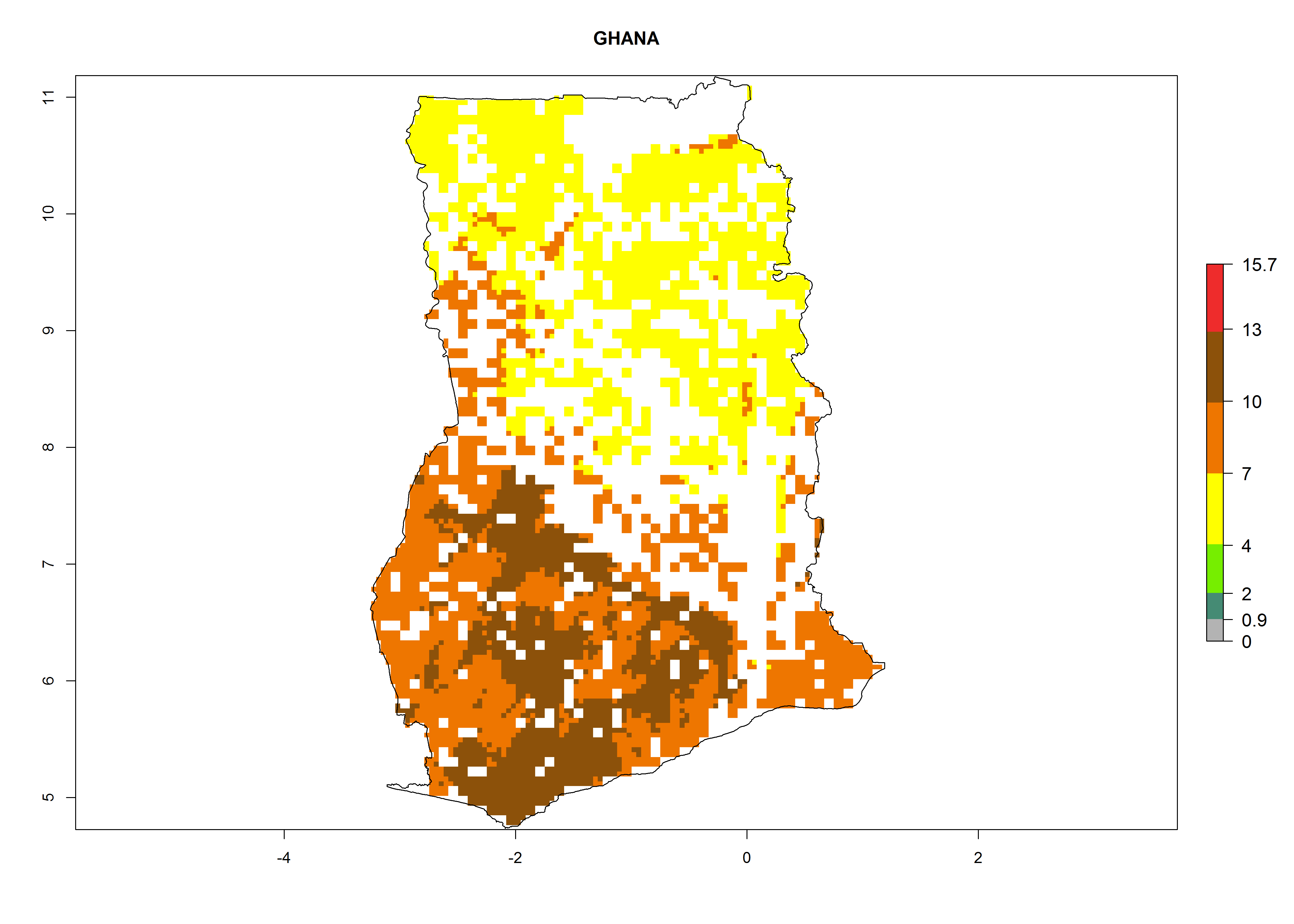
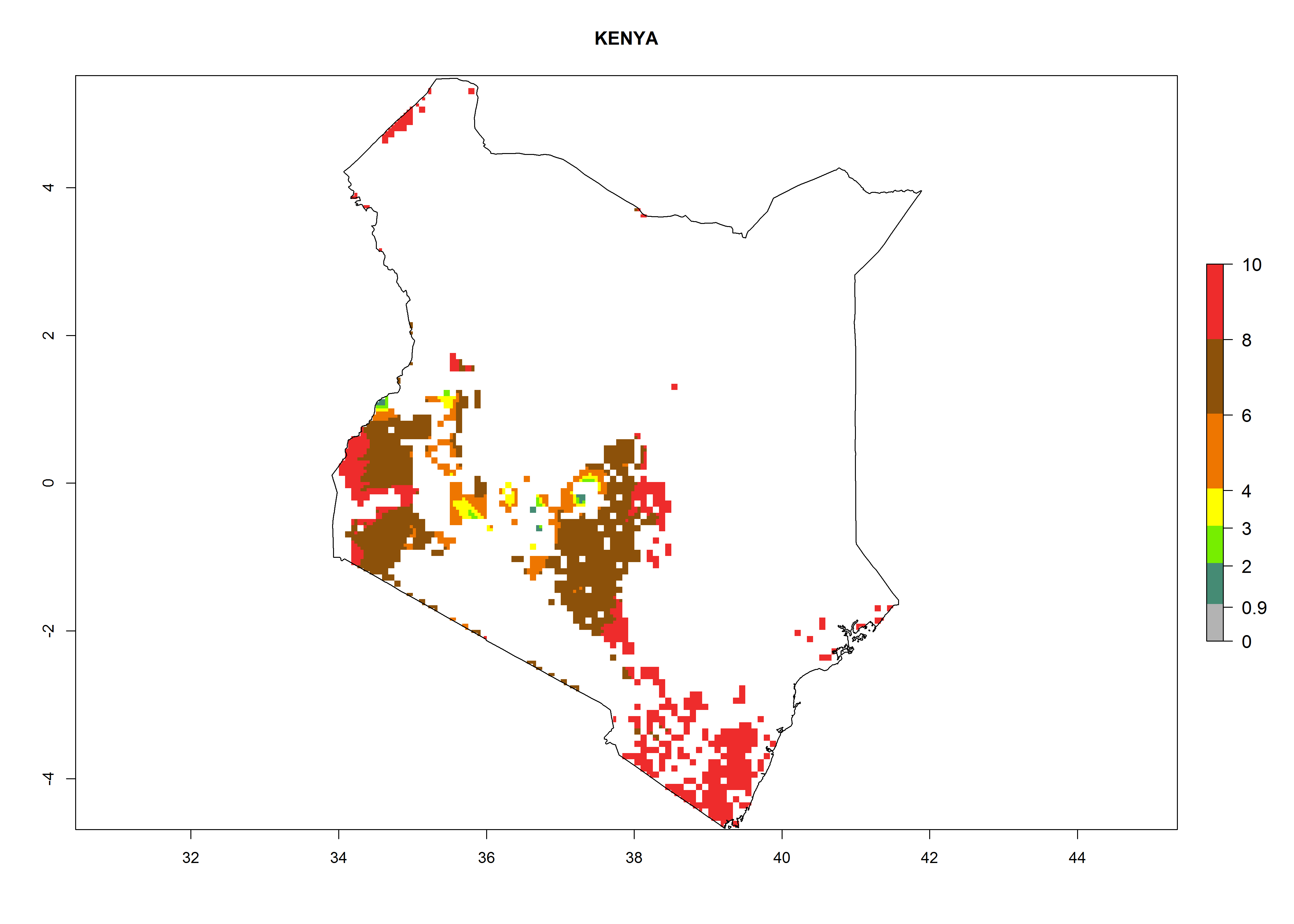
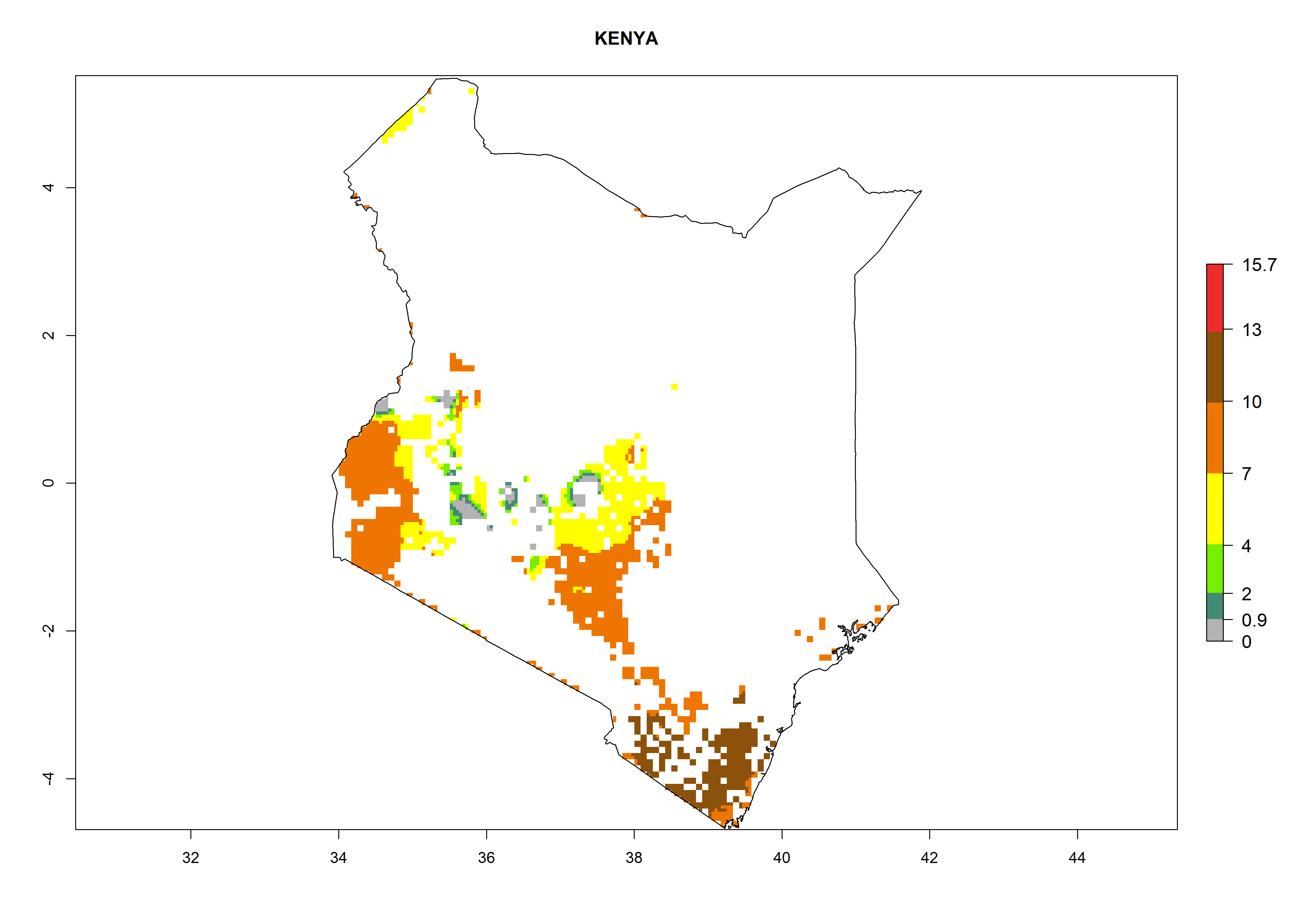
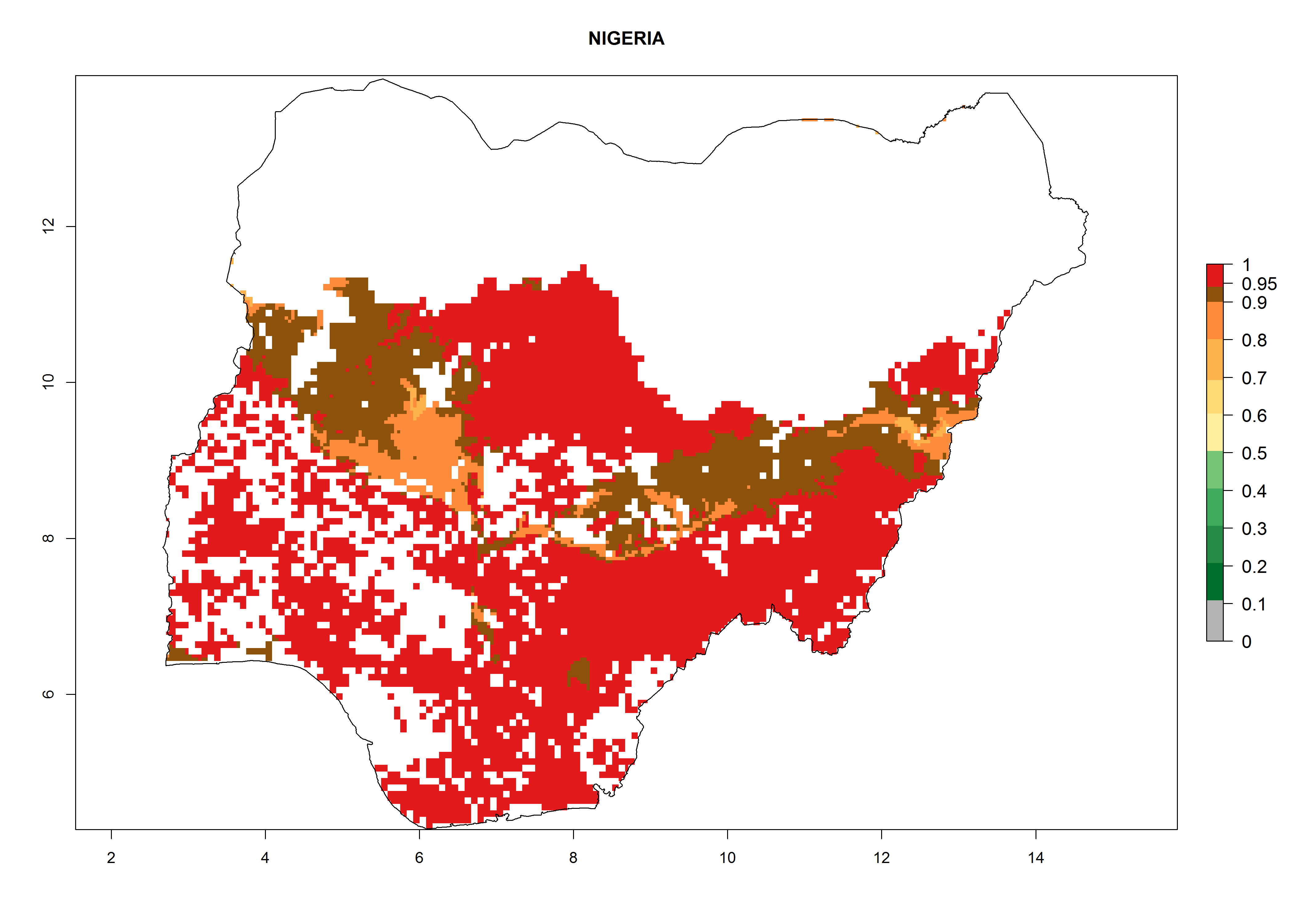
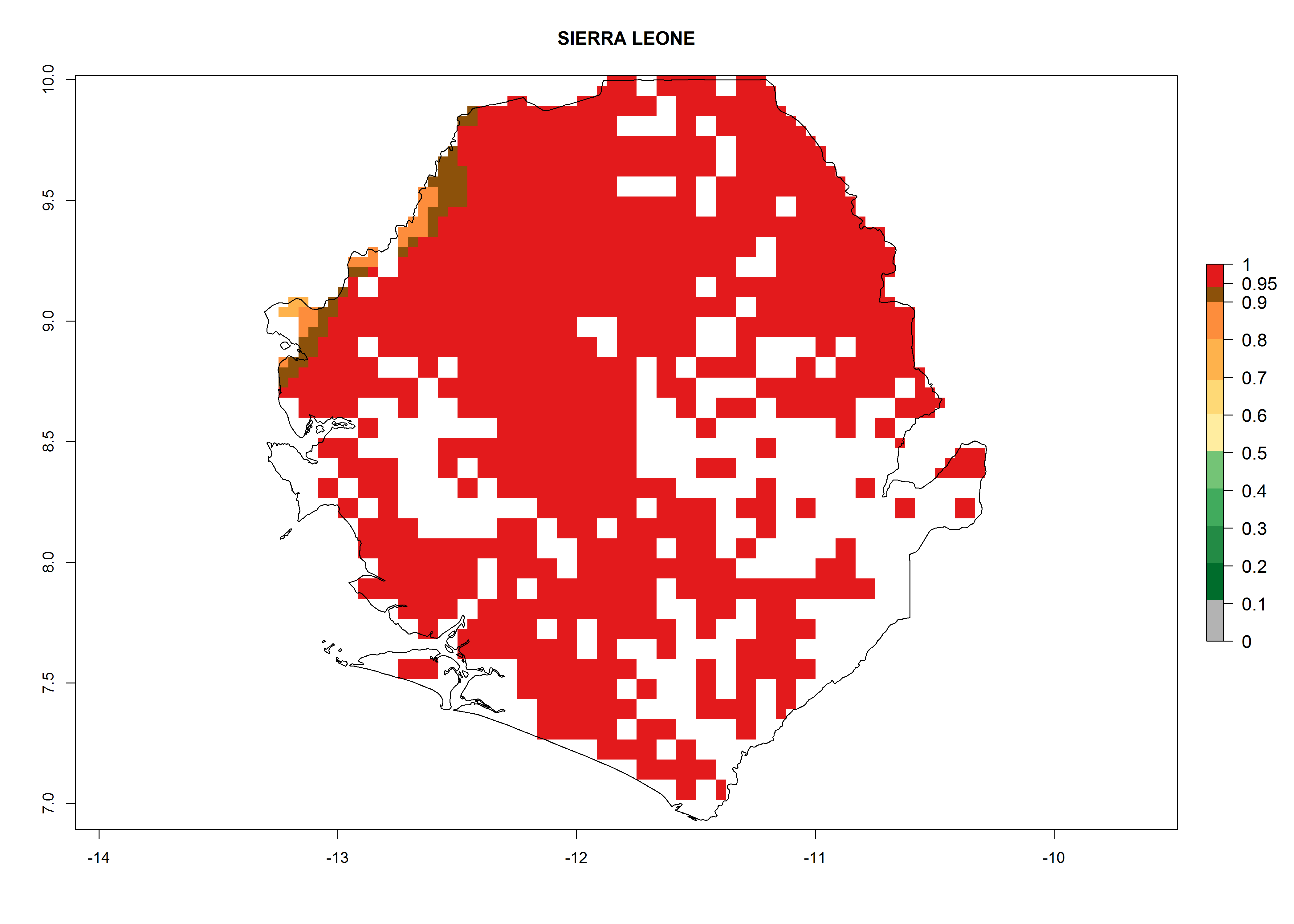
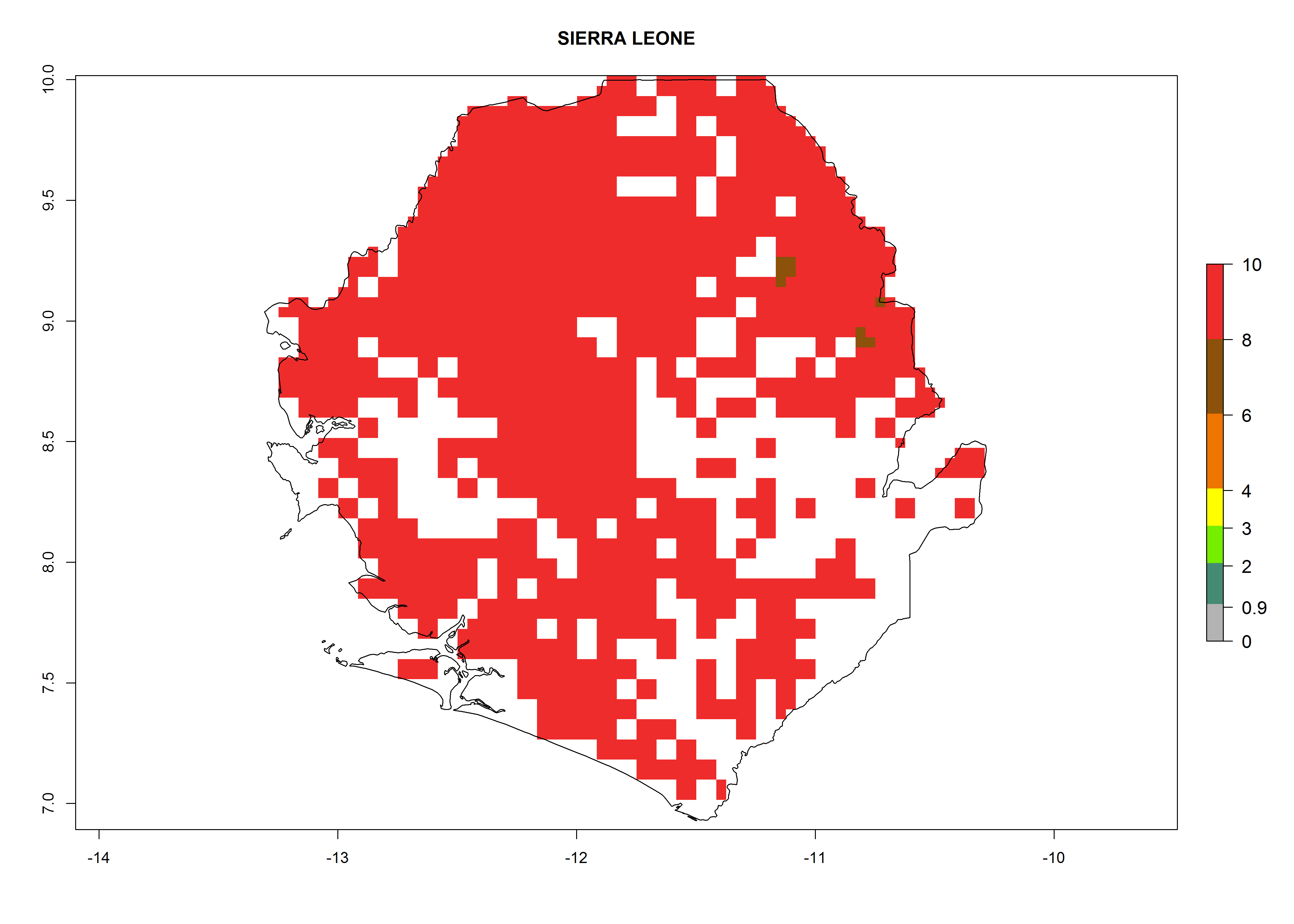
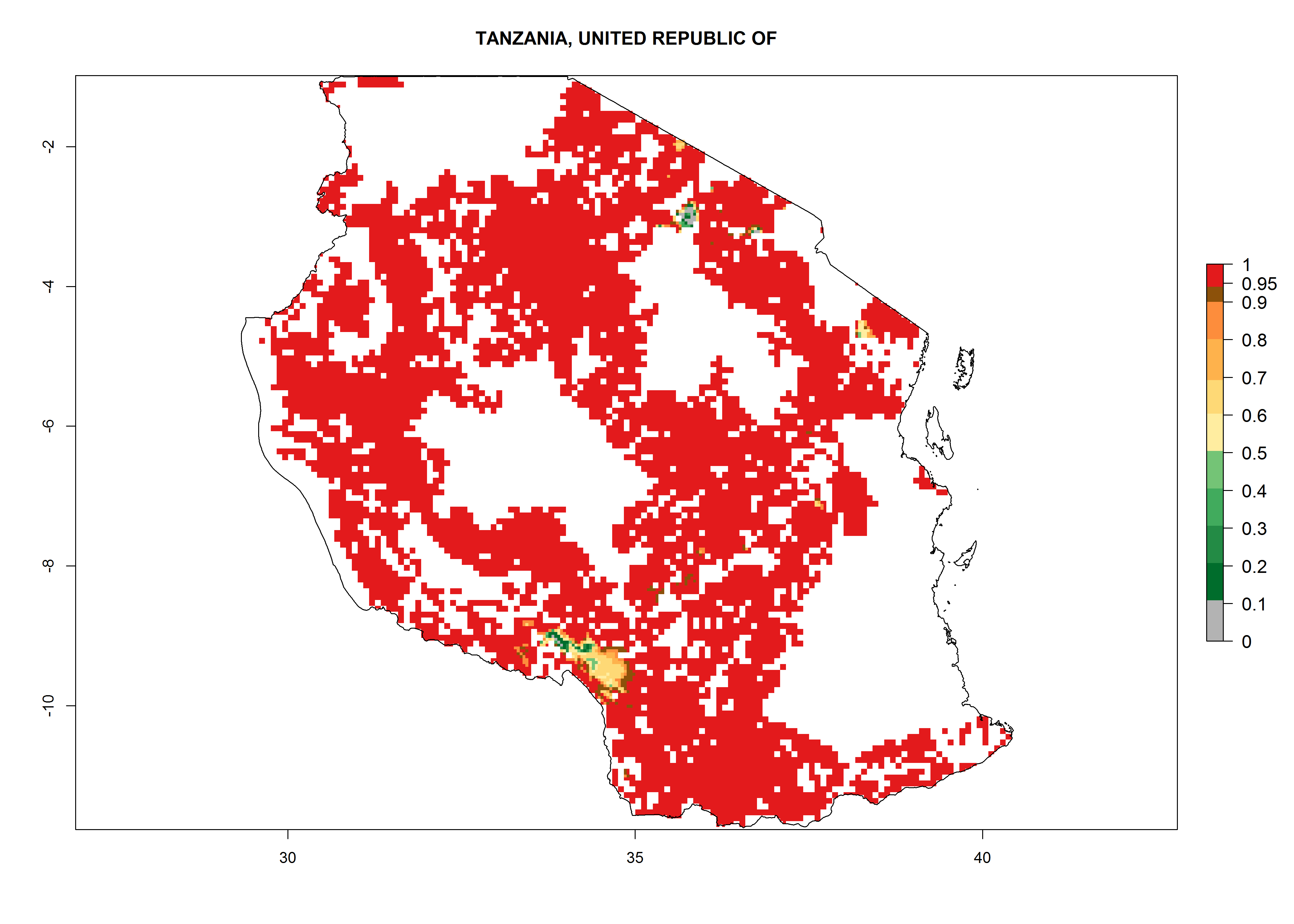
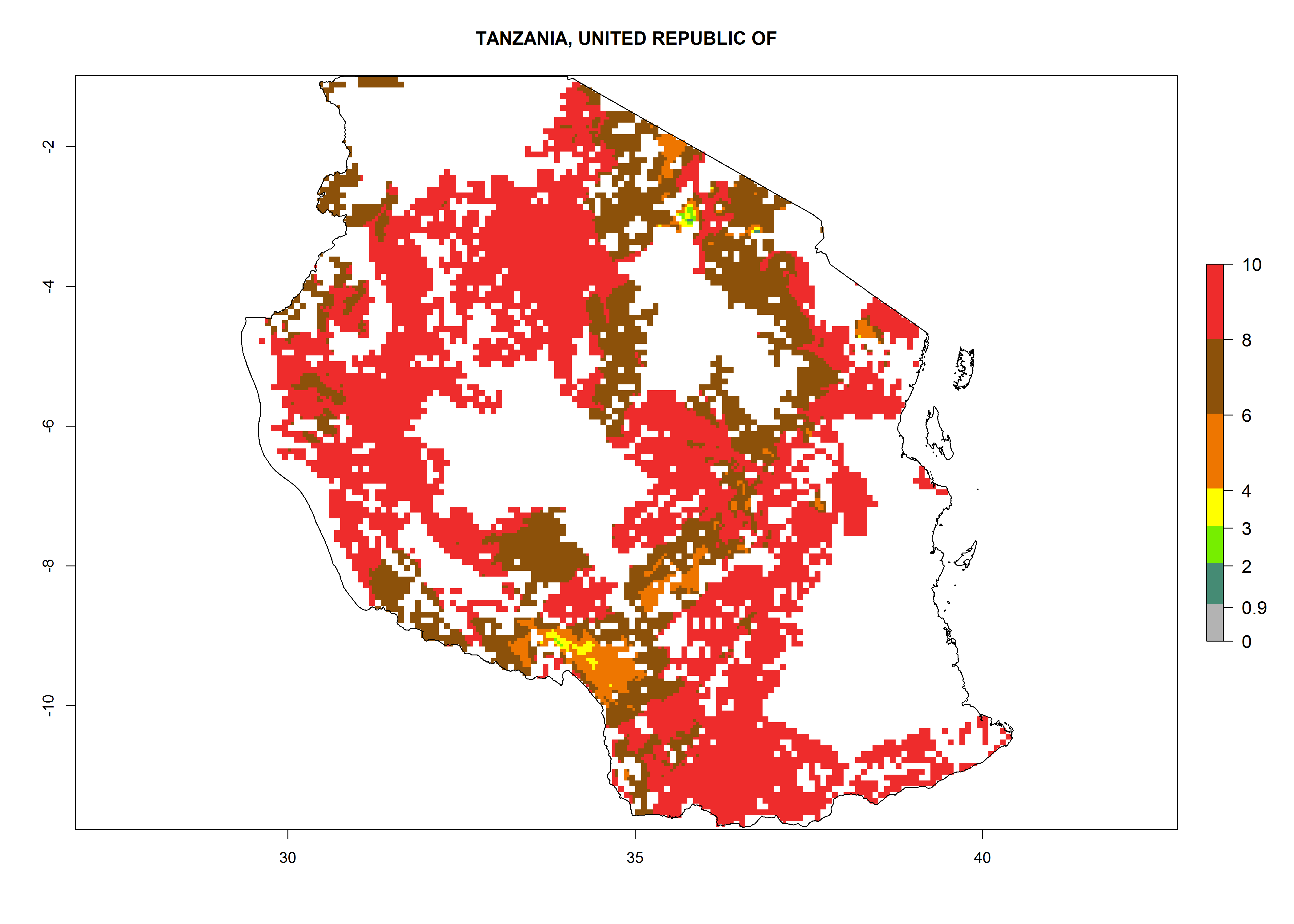
Under the current climate, B. afer represents a severe risk (ERI>0.95) in East and West Africa, which is associated with a GI>6–10 generations per year and a high AI>7 (Fig. 6). The highest risks of establishment (ERI≥0.95, red zones) are observed in lowland production zones of each country (Fig. 7). Countries under potential risk of B. afer under the present climate are Angola, Burkina Faso, Sudan, Uganda, Ethiopia, Kenya, Tanzania, Malawi, Mozambique, Madagascar, Kenya, South Africa, Tanzania, and Mozambique. In the case of Tanzania, a large part of the country is situated at an altitude of 1,000 masl, mostly characterized by a temperate tropical warm climate. Tanzania presents the largest area with high risk of establishment of B. afer. It is known to suffer from damaging whitefly-transmitted diseases in sweetpotato and cassava. For the northeastern zones of Africa (Sudan), it is predicted that B. afer develops 8 generations per year with an AI>4 (Fig. 7o). In Southern Africa (South Africa) an ERI>0.6 is projected (Fig. 7n).
Figure 7. ERI, abundance (GI, potential damage), and activity (AI, potential population growth) of the whitefly, Bemisia afer, in sweetpotato production regions of selected African countries according to model predictions for the year 2000. An ERI>0.95 is associated with potential permanent establishment.
Phytosanitary measures
In countries where B. afer is not already present, the enforcement of strict phytosanitary regulations may help to reduce the risk of this whitefly becoming established. Because of the difficulty of detecting low levels of infestation in consignments, it is best to ensure that the place of production is free of the pest. B. afer is on the European Plant Protection Organisation alert lists.
Adaptation to risk avoidance at farm level
This requires the correct design and implementation of integrated pest management measures. Few studies have been carried out on B. afer specifically, but it is assumed that similar measures as applied to other whitefly species can be used (see section 4.2.4).
Further reading
Abd-Rabou, S. 2007. Host plants, geographical distribution and natural enemies of the sycamore whitefly, Bemisia afer (Priesner & Hosny), a new economic pest in Egypt. In: Fourth International Bemisia Workshop International Whitefly Genomics, Dec. 3–8, 2006, Duck Key, Florida, USA. 2pp. Available online: insectscience.org/8.04
Anderson, P., J. Martin, P. Hernandez, and A. Lagnaoui. 2001. Bemisia afer ses. lat. (Homoptera: Aleyrodidae) outbreak in the Americas. Florida Entomologist 84: 316–317.
Chu, D., G. Liu, F. Wan, Y. Tao, and R.J. Gill. 2010. Phylogenetic analysis and rapid identification of the whitefly Bemisia afer in China. Journal of Insect Science 10(86): 1–9.
Cisneros, F., and N. Mujica 1999. Biological and selective control of the sweetpotato whitefly, Bemisia tabaci (Gennadius) (Hom.: Aleyrodidae). Program report, 1997–98. International Potato Center (CIP), Lima, Peru, pp. 255–264.
European Plant Protection Organisation (EPPO). 2006. EPPO reporting service. Pest and diseases. No. 11. 17 pp.
Evans, G.A. 2007. The whiteflies (Hemiptera: Aleyrodidae) of the world and their host plants and natural enemies. Version 070606. Last Revised: June 2007: 234–683.
Gamarra, H.A., S. Fuentes, F.J. Morales, R. Glover, C. Malumphy, and I. Barker. 2010. Bemisia afer sensu lato, a vector of Sweet potato chlorotic stunt virus. Plant Diseases 94: 510–514.
Lagnaoui, A., Mujica, N., Zegarra O., and P. Anderson. 2001. The whitefly complex (Homoptera: Aleyrodidae) affecting sweetpotato in the Canete Valley of Peru. ISHS CIP. Sweetpotato: Food and health for the future, an international symposium, guide for participants. Lima, Peru. ISHS; BII-P-11.
Maruthi, M.N., S. Navaneethan, J. Colvin, and R.J. Hillocks. 2004. Bionomics, morphometric and molecular characterization of a cassava Bemisia afer (Priesner & Hosny) population. International Journal of Tropical Insect Science 24(4): 323–329.
Maruthi, M.N., A.R. Rekha, P. Sseruwagi, and R.J. Hillocks. 2006. Mitochondrial DNA Variability and Development of a PCR Diagnostic Test for Populations of the Whitefly Bemisia afer (Priesner and Hosny). Molecular Biotechnology 34: 10.
Morales, F.J., C. Cardona, J.M. Bueno, and I. Rodríguez. 2006. Manejo Integrado de enfermedades de plantas causadas por virus transmitidos por moscas blancas. Centro Internacional de Agricultura Tropical (CIAT), Cali, Colombia. 43 pp.
Mujica, N., Marchena, M., Fabian, F., and A. Lagnaoui. 2001. Integrated management of the sweetpotato whitefly, Bemisia tabaci, in commercial sweetpotato production. ISHS CIP. Sweetpotato: Food and health for the future, an international symposium, guide for participants. Lima, Peru.
Navas-Castillo, J., E. Fiallo-Olivé, and S. Sánchez-Campos. 2011. Emerging virus diseases transmitted by whiteflies. Annual Review Phytopathology 49: 219–248.
Rathore, Y.S., and S.N. Tiwari. 2015. Influence of taxonomic diversity on host plant preference of different species of Bemisia. International Journal of Agricultural Sciences and Veterinary Medicine 3(2): 50–63.
Untiveros, M., S. Fuentes, and L.F. Salazar. 2007. Synergistic interaction of Sweet potato chlorotic stunt (Crinivirus) with carla-cucumo-, ipomo- and potyviruses infecting sweet potato. Plant Disease 91: 669–676.