5.1.3 Biocontrol Agents Associated With Potato And Vegetable Pests![]() / Orgilus lepidus (Muesebeck 1967)
/ Orgilus lepidus (Muesebeck 1967)
Taxonomic position: Hymenoptera, Braconidae (Orgilinae)
Authors: V. Cañedo, P. Carhuapoma, C. Bartra, & J. Kroschel
Hosts
Potato tuber moth, Phthorimaea operculella (Zeller); Andean potato tuber moth, Symmetrischema tangolias (Gyen); Guatemalan potato tuber moth, Tecia solanivora (Povolny)
Morphology
Egg
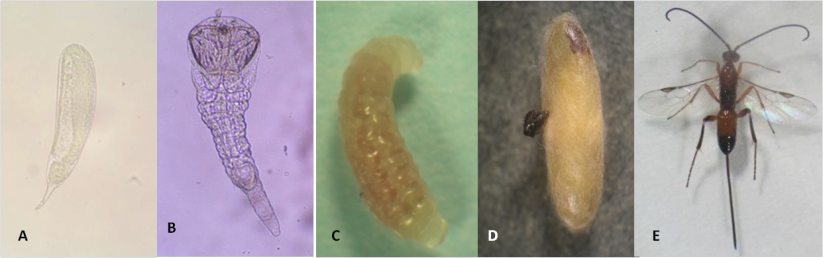
Elongated and translucid with a small pedicel about 0.38 x 0.13 mm in size (Photo 1A).
Larva
It has three instars. The first instar is mandibulate-caudate type with 13 body segments and measures approximately 0.46 x 0.16 mm (Photo 1B). The third instar is hymenopteriform and measures 3.01 x 0.92 mm (Photo 1C).
Pupa
The pupa is exarate type (appendages are free and not glued to the body) about 4.3 x 1.1 mm in size. It develops inside the cocoon made by the mature last instar larva, soon after it emerges from the host (Photo 1D).
Adult
The adult is yellowish-brown. The two sexes appear quite similar except that the female has a long straight ovipositor, which is about the same length as the body from the tip of the head to the end of the abdomen (Photo 1E). The mean length of the ovipositor is 4.5 mm. The wasp is a good flyer and is able to search and host larvae in dense foliage.
Biology
Parasitism
Orgilus lepidus females lay their eggs in early instar potato tuber moth larvae. Females fly preferentially to either mechanically damaged plants or to plants infested by P. operculella larvae, rather than to undamaged plants. Also, females are attracted by the odor of plants, especially damaged ones. Females spent more time probing with their ovipositor on leaves infested by their host instead of on mechanically damaged plant leaves. Wasps responded similarly to leaves infested with hosts and leaves from which hosts were removed, indicating that plant damage caused by their host or host products are the primary clues used to discriminate different kinds of damage and locate their hosts. Host-finding by O. lepidus is mediated by two kairomones present in the frass of the host insect: (1) a volatile substance identified as heptanoic acid elicits heightened searching behavior, resulting in the parasite contacting the frass, and (2) a contact chemical stimulus in the frass induces the parasite to probe the substrate with the ovipositor, culminating in contact with the host. After parasitizing P. operculella larvae, the wasp eggs hatch inside and develop into fully formed wasps in about 21 days, depending on the temperature. Unfertilized females produce only males (arrhenotoky parthenogenesis).
Temperature-dependent development
O. lepidus completed its development from egg to adult in temperatures between 15°C and 30°C (see Annex 7.4.3). Total development at 15°C was almost five times longer (85 days) than at 30°C (16 days). Its full life cycle could not be completed at 11°C. The highest mortality of egg-larvae was 37% at 15°C and around 14% at temperatures of 20°–30°C. Pupae mortality was highest at extreme temperatures, with 44% and 56% at 15°C and 30°C, compared with 13% at temperatures of 20°–25°C, respectively. Female longevity decreased with high temperatures, from 22 days (15°C) to 3 days (30°C). The optimum temperature for reproduction ranged from 25° to 30°C. The maximum fecundity of 70 offspring was observed in the temperature range of 25°–28°C. The sex ratio was not affected by temperature (1:1).
The established functions were used to estimate the life-table parameters of O. lepidus and to build an overall stochastic phenology model. Negative r values below 15°C indicate that population size is decreasing at these temperatures; positive r values between 18°C and 32°C indicate that the population is increasing. The finite rate of increase peaked at 28°C (λ=1.13) and was smallest at 11°C (λ=0.98); λ<1 indicates that the population is decreasing. Highest values for the gross reproduction rate and net reproductive rate (Ro) were found at 25°–28°C. The shortest mean generation time (T) and doubling time (Dt) was observed at 28°C (20.38 days and 5.72 days, respectively). The optimum temperature for overall population growth ranged between 25°C and 28°C. On the basis of the characteristics of fecundity, daily oviposition, and longevity, O. lepidus can be considered as having good potential to control P. operculella populations (see Annex 7.4.3).
Economic impact in pest control
O. lepidus has been used in classical biological control programs in several countries, often in combination with the braconid A. subandinus and the encyrtids Copidosoma koehleri (see sections 5.1.1 and 5.1.2). In Australia, all three species were recognized as an important mortality factor of P. operculella. And although high parasitism rates occurred, high crop damage was still observed. O. lepidus was better adapted to warm coastal areas than to the cooler and drier inland areas of Australia and successfully coexisted with A. subandinus; however, it replaced A. subandinus in coastal New South Wales. Parasitism rates exceeded 50%, and introduced C. koehleri and O. lepidus often accounted for 92.6% of total parasitism.
Geographical distribution
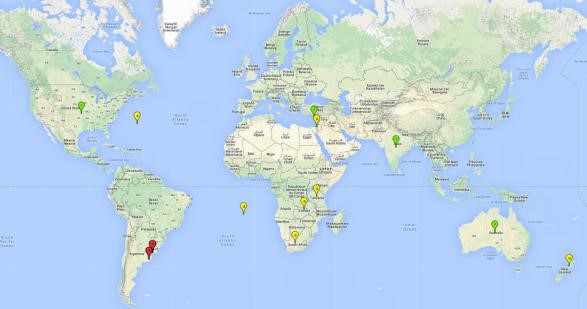
Possible region of origin: South America: Argentina and Uruguay
Introduced and established: Australia, Cyprus, India, USA
Introduced but establishment not confirmed: Bermuda, Israel, New Zealand, St. Helena, South Africa, Tanzania, and Zambia (Fig.1).
Potential establishment and efficiency under current and future climates
Changes in global establishment and future distribution
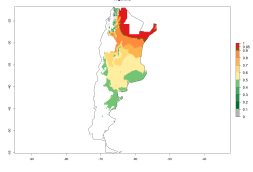
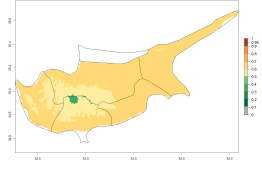
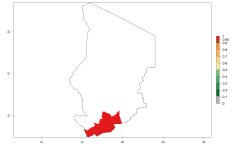
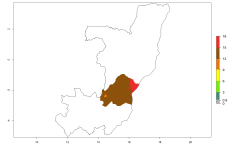
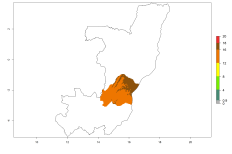
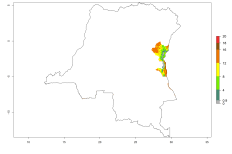
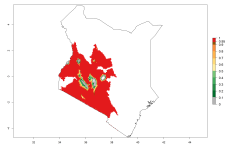
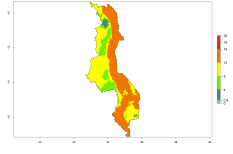
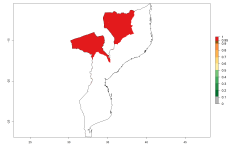
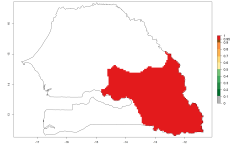
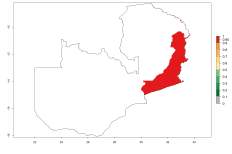
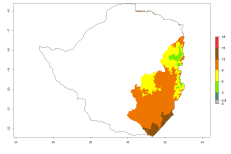
In potato production regions globally, the potato tuber moth, P. operculella, is potentially established at an establishment risk index (ERI)>0.7 (see section 4.1.1). Potential release regions for O. lepidus are those potato production regions where the potato tuber moth has been established, causing significant economic damage in potato fields and stores. Therefore, the establishment index (EI) for the parasitoid O. lepidus is only displayed for those regions where P. operculella can potentially establish. An EI=1 indicates survival of the parasitoid throughout the year—that is, the likelihood of long-term establishment for classical biological control is very high in these regions. However, O. lepidus has also been established in regions with an EI>0.5–0.7, indicated by creamy-colored zones in Argentina, Cyprus, and Australia (Fig. 2; compare with Fig. 1).
| Argentina | Cyprus | Australia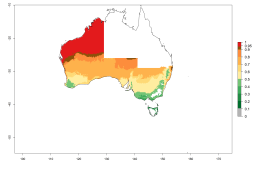 |
Figure 2. EI of Orgilus lepidus in countries and regions where establishment has been reported according to model predictions of the year 2000. An EI>0.5 (light yellow to red zones) indicates regions with permanent establishment.
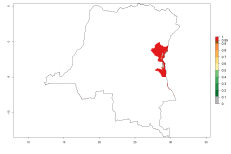
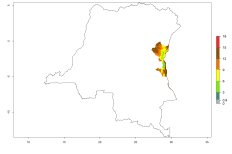
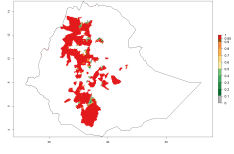
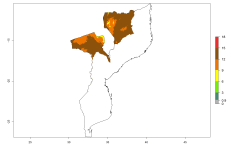
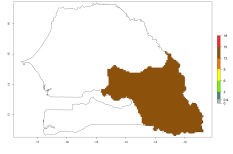
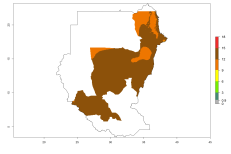
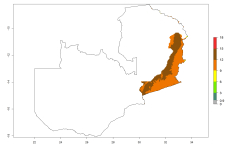
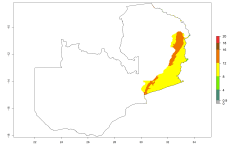
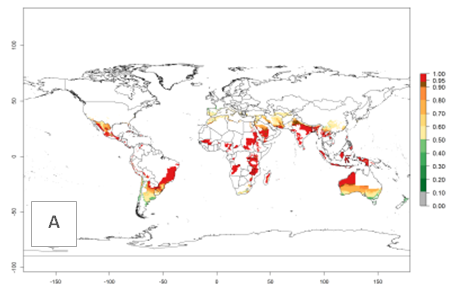
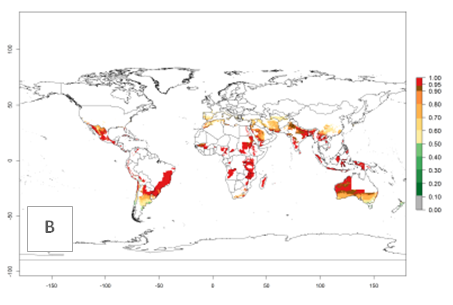
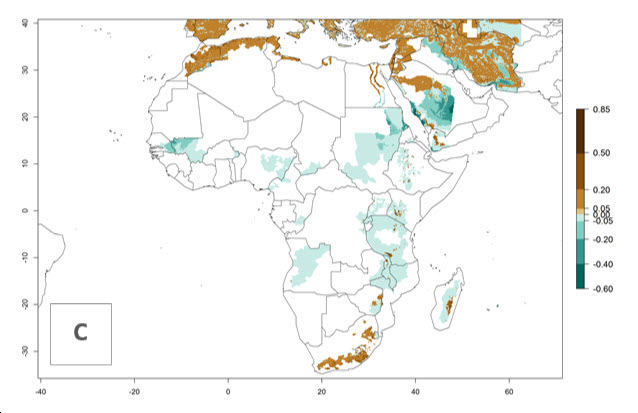
Many regions can be identified where O. lepidus has an EI>0.8. These are located in South America (Venezuela, Ecuador, Peru, southeast of Brazil, Bolivia); Central America (Panama, Costa Rica, Nicaragua, Mexico); in potato-growing areas of countries in East Africa (e.g., Tanzania); and in Asia (India, Indonesia, Australia) (Fig. 3A). Global predictions for 2050 show that the establishment of O. lepidus will potentially increase (EI>0.95) in some potato-growing areas of South America (Ecuador, Peru, Chile, Bolivia, Brazil); in some countries of West and Southern
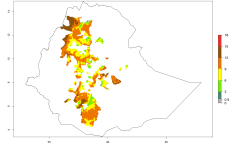
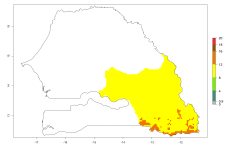
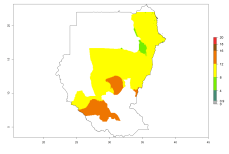
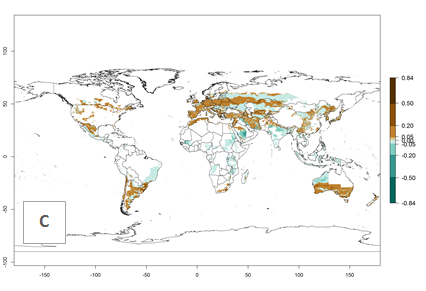
Africa; and in southern Australia. A slight range expansion is projected to more temperate regions of southern South America (Fig. 3B). In contrast, the potential establishment decreases in some potato-growing areas of India and some regions of northwest Australia (Fig. 3C).
 |
 |
 |
|
Figure 3. Changes in establishment and potential distribution worldwide of Orgilus lepidus according to model predictions, using the EI for the years 2000 (A), and 2050 (B) and changes of the EI between 2000 and 2050 (C) displayed in potato production regions with an ERI>0.7 of its primary host Phthorimaea operculella. An EI>0.5 indicates regions with potential permanent establishment.
Changes in global abundance
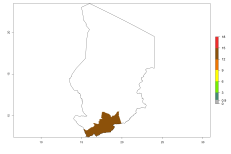
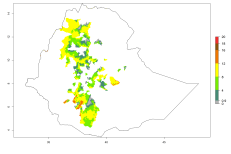
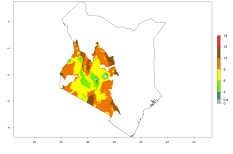
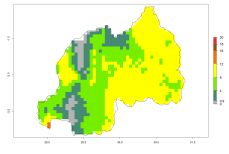
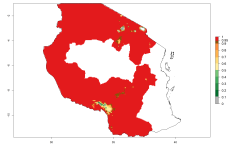
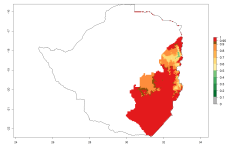
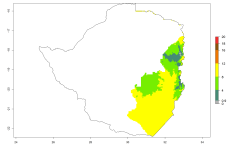
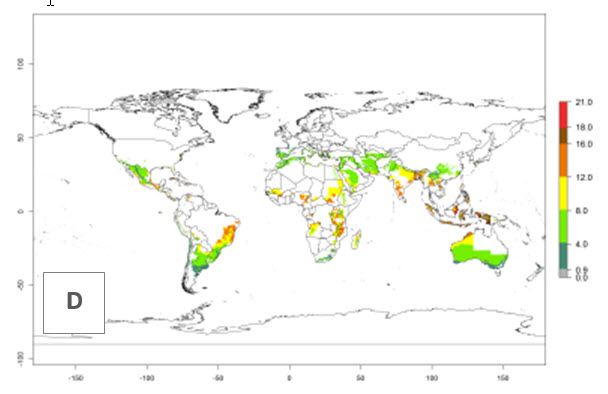
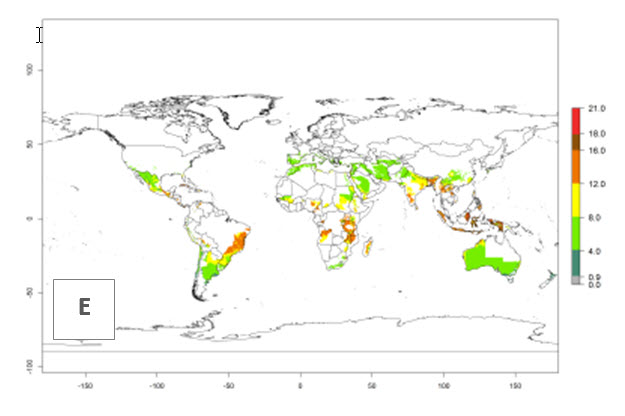
The generation index (GI) reflects the potential abundance of the parasitoid population, and it estimates the mean number of generations that O. lepidus may produce in potato production regions worldwide according to existing and predicted temperature conditions of the years 2000 and 2050 (Fig. 4A, B). The number of generations in countries where O. lepidus is established today ranges from 6 to 9 per year. For the year 2050, in some tropical areas of America (Mexico, Peru, Ecuador, Bolivia, Chile, southeast Brazil); Southern and East Africa; and Indonesia, the generation change index indicates a potential increase of 2–4 generations per year (Fig. 4C).
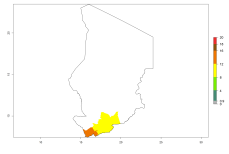
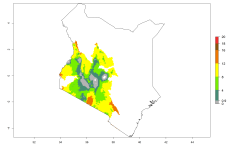
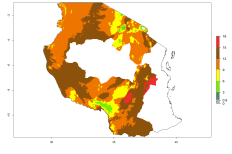
The activity index (AI) highlights not only the establishment but also the potential spread of an insect and, in the case of parasitoids, its potential to efficiently control its host. For the year 2000, an AI of 4–8 is indicated for those countries where O. lepidus is present today (Fig. 4D). Predictions of change for the 2050 climate change scenario show an increase in the potential growth by a factor of 1–5 in some potato-growing areas of Peru, Bolivia, Chile, Argentina, and southeast Brazil in South America; in countries of Southern and East Africa; some regions of South Asia; and southern Australia. In contrast, a reduction of the potential growth will be expected in some potato- growing areas of northwestern Australia (Fig. 4E, F).
| GI | AI | |
| 2000 | 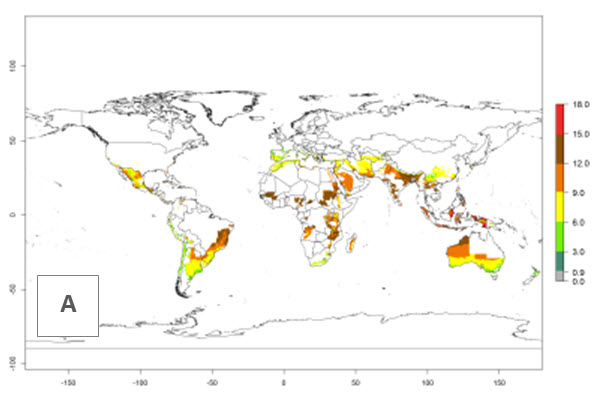 |
 |
| 2050 | 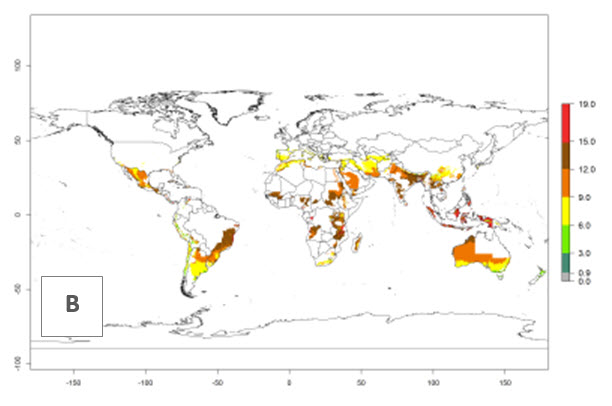 |
 |
| Index change (2000 – 2050) | 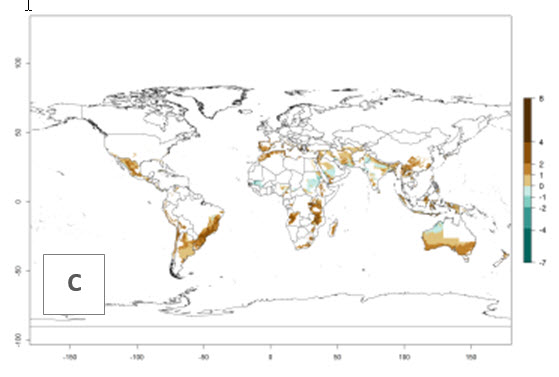 |
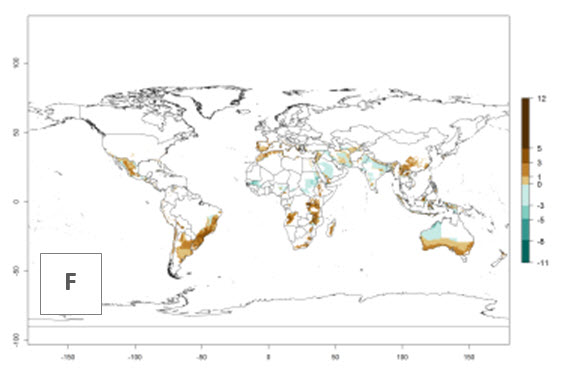 |
Figure 4. Changes in abundance (GI, number of generations/year) and activity (AI, potential population growth) of Orgilus lepidus according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F), displayed in potato production regions with an ERI>0.7 of its primary host Phthorimaea operculella.
Changes in regional establishment and distribution in Africa
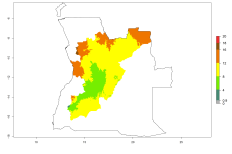
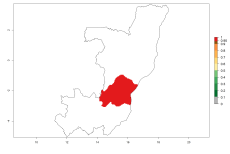
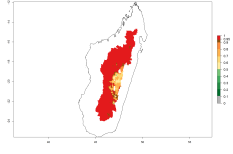
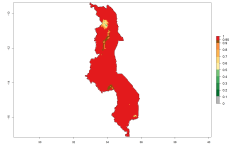
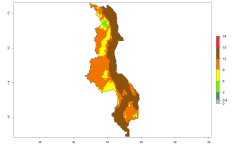
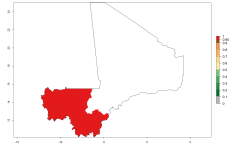
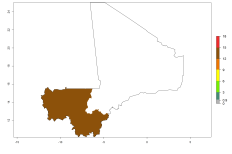
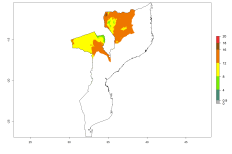
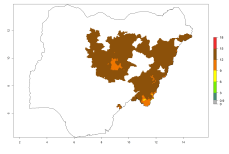
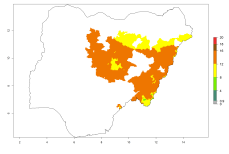
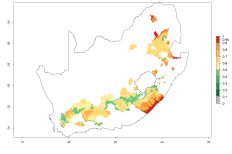
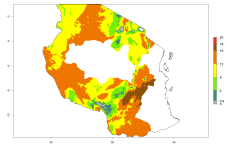
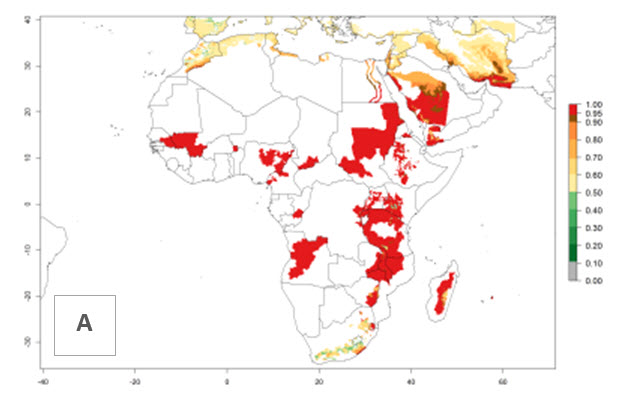
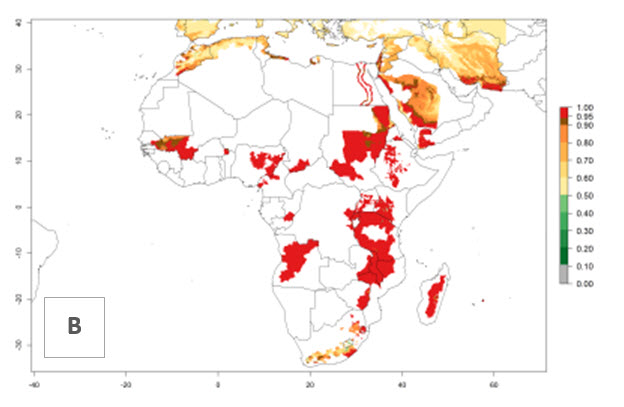
In Africa, O. lepidus has been introduced in South Africa, Tanzania, and Zambia, but its establishment was not further confirmed. According to the mapping results, however, a successful establishment could have been expected in those countries. In some tropical countries of Africa, an EI>0.9 indicates a very high potential establishment of O. lepidus according to current temperature conditions in the potato-growing areas of Angola, Cameroon, Chad, Congo, DR Congo, Ethiopia, Kenya, Madagascar, Malawi, Mali, Mozambique, Niger, Nigeria, Rwanda, Senegal, South Africa, Sudan, Tanzania, Uganda, Zambia, and Zimbabwe (Fig. 5A). Owing to climate change, it can be expected that the establishment will potentially increase in some other regions of Kenya, Madagascar, and South Africa. In contrast, the establishment will potentially decrease in some regions of Senegal, Mali, and Sudan (Fig. 5B, C).
 |
 |
 |
|
Figure 5. Changes in establishment and potential distribution of Orgilus lepidus in Africa according to model predictions, using the EI for the years 2000 (A) and 2050 (B) and changes of the EI between 2000 and 2050 (C), displayed in potato production regions with an ERI>0.7 of its primary host Phthorimaea operculella. An EI>0.5 indicates regions with permanent establishment.
Changes in regional abundance in Africa
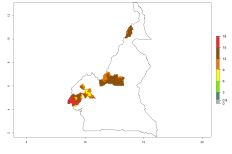
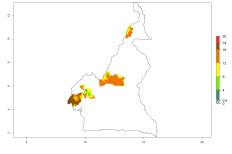
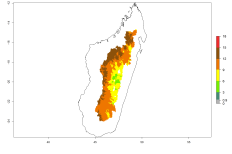
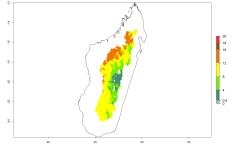
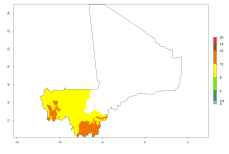
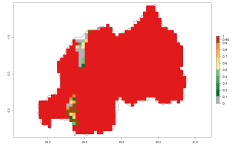
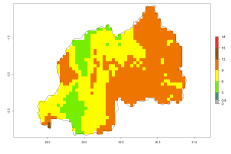
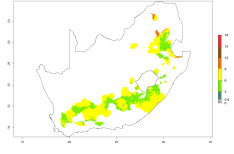
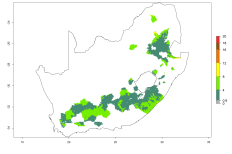
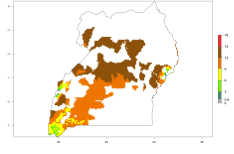
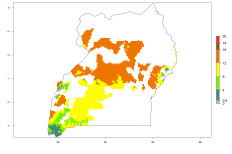
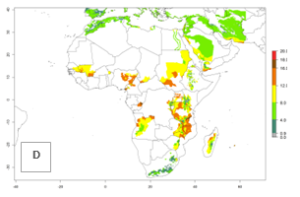
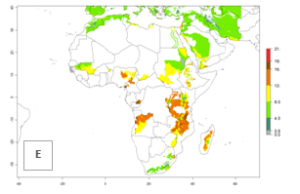
In many potato-growing areas of Africa, the GI reveals 9–15 generations per year (Fig. 6A). Predictions for the 2050 scenario show an increase in generation numbers per year in Angola, Cameroon, Congo, Ethiopia, Kenya, Madagascar, Malawi, Mozambique, Rwanda, South Africa, Tanzania, Uganda, Zambia, and Zimbabwe (Fig. 6B, C). The GI is strongly correlated with the AI. The AI is expected to increase in some potato-growing areas of Congo, Angola, South Africa, and countries of East Africa. This is especially the case in Uganda, Tanzania, Zimbabwe, Malawi, Mozambique, and Madagascar, where a high O. lepidus AI>12 is projected (Fig. 6D–F).
| GI | AI | |
| 2000 | 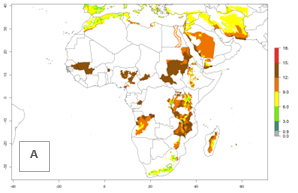 |
 |
| 2050 | 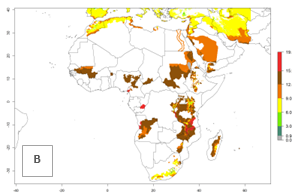 |
 |
| AI | 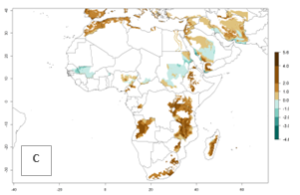 |
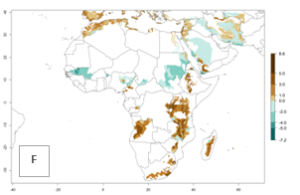 |
Figure 6. Changes in abundance (GI, number of generations/year) and activity (AI, potential population growth) of Orgilus lepidus in potato production regions of Africa according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F), displayed in potato production regions with an ERI>0.7 of its primary host Phthorimaea operculella.
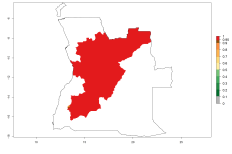
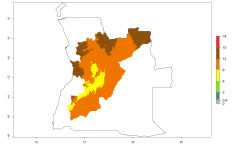
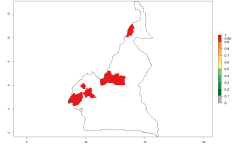
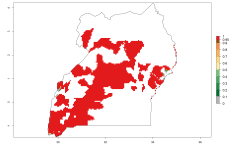
Potential release areas in Africa
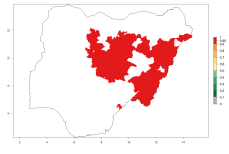
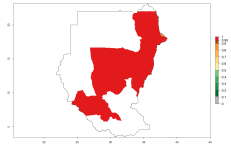
Considering an ERI>0.7 for P. operculella in potato-growing areas in Africa, the potential countries to release O. lepidus under the current climate are Angola, Cameroon, Chad, Congo, DR Congo, Ethiopia, Kenya, Madagascar, Malawi, Mali, Mozambique, Nigeria, Rwanda, Senegal, South Africa, Sudan, Tanzania, Uganda, Zambia, and Zimbabwe (Fig. 7). O. lepidus has been shown to establish at an EI>0.5; in all suggested countries the likelihood of establishment is expected to be higher in almost all potato production regions (EI>0.9). This high establishment potential is associated with a GI>9 (i.e., more than 9 generations potentially develop per year) and an AI>8.
Figure 7. EI, abundance (GI, number of generations/year), and activity (AI, potential population growth) of Orgilus lepidus in selected African countries according to model predictions for the year 2000, displayed in potato production regions with an ERI>0.7 of its primary host Phthorimaea operculella. An EI>0.5 indicates regions with permanent establishment.
Risks to no-targets
No risks are reported. Species of the potato tuber moth complex are the only hosts of O. lepidus, and little interference with other species is expected after its introduction into a new area or region. In regions where P. operculella has been accidently introduced, generally local parasitoids specific to P. operculella are absent. In such situations, local parasites comprise generally oligo- or polyphagous species. O. lepidus might dominate local species because it is more specific to the moth.
Further reading
Bartra, M.C. 2009. Estudios biológicos y efecto de la temperatura sobre Orgilus lepidus Muesebeck (Hymenoptera: Braconidae), parasitoide de la polilla de la papa Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae). BSc thesis, Universidad Nacional Federico Villarreal, Lima, Peru.
Callan, E.M. 1974. Changing status of the parasites of potato tuber moth Phthorimaea operculella (Lepidoptera:Gelechiidae) in Australia. Entomophaga 19: 97–101.
Hendry, L.B., P.D. Greany, and R.J. Gill. 1973. Kairomone mediated host-finding behavior in the parasitic wasp Orgilus lepidus. Entomologia Experimentalis et Applicata 16: 471–477.
Hendry, L.B., J.K. Wichmann, D.M. Hindenlang, K.M. Weaver, and S.H. Korzeniowski. 1976. Plants—the origin of kairomones utilized by parasitoids of phytophagous insects? Journal of Chemical Ecology 2: 271–283.
Horne, P.A. 1990. The influence of introduced parasitoids on the potato moth, Phthorimaea operculella (Lepidoptera: Gelechiidae) in Victoria, Australia. Bulletin of Entomological Research 80: 159–163.
Horne, P.A., and J. Page. 2008. IPM dealing with potato tuber moth (PTW) and all other pests in Australian potato crops. In Integrated Pest Management for the Potato Tuber Moth, Phthorimaea operculella Zeller – a Potato Pest of Global Importance. J. Kroschel and L. Lacey eds., 111–117. Tropical Agriculture 20, Advances in Crop Research 10. Weikersheim, Germany: Margraf Publishers.
Keller, M.A., and P.A. Horne. 1993. Sources of host-location cues for the parasitic wasp Orgilus lepidus (Braconidae). Australian Journal of Zoology 41: 335–341.
Oatman, E.R., and G.R. Platner. 1974. Parasitization of the potato tuberworm in southern California. Environmental Entomology 3: 262–264.
Salehi, L. 2003. A study on reproductive capacities and rate in parasitism of two parasitoids of potato tuber moth. Iranian Journal of Agricultural Sciences 34: 231–245.