5.1.5 Biocontrol Agents Associated With Potato And Vegetable Pests![]() / Phaedrotoma scabriventris (Nixon 1955)
/ Phaedrotoma scabriventris (Nixon 1955)
Synonyms: Opius scabriventris (Nixon 1955)
Opius (Opius) scabriventris (Nixon 1955)
Opius (Gastrosema) scabriventris (Nixon 1955)
Taxonomic position: Hymenoptera, Braconidae (Opiinae)
Authors: N. Mujica, C. Valencia, P. Carhuapoma, & J. Kroschel
Hosts
Phaedrotoma scabriventris parasitizes leafminer species (Diptera: Agromyzidae) of economic importance: Liriomyza huidobrensis Blanchard, L. brassicae Riley, L. sativae Blanchard, and L. trifolii (Burgess) in vegetables. P. scabriventris has also been recovered from leafminer flies of minor importance infesting wild plants as Calycomyza cruciata Valladares, C. humeralis Roser, C. lantanae Frick, C. malvae Burgess, C. verbenivora Spencer, Chromatomyia platensis Brethes, Haplopeodes cordobensis Valladares, H. lycivora Valladares, Haplopeodes spp., Liriomyza cesalpiniae Valladare, L. commelinae Frost, L. near to sabaziae, Phytomyza crassisseta Zetterstedt, and P. williamsoni Blanchard.
Morphology
Egg
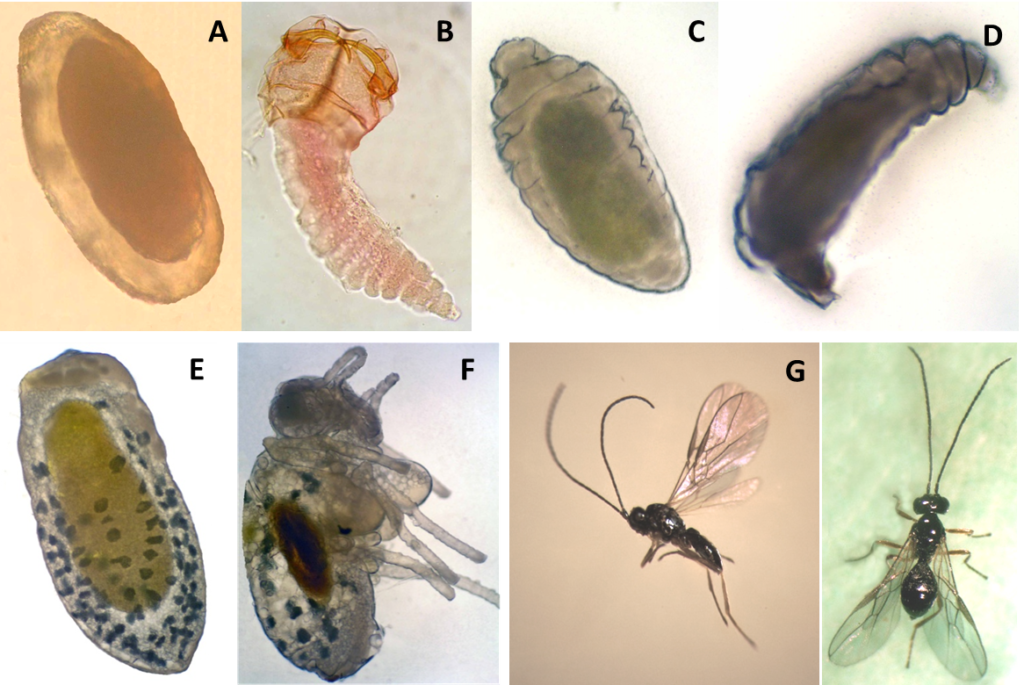
Hymenoptera in form and monoembryonic, oval and elongated, slightly widening toward the back (0.1006 x 0.0478 mm) (Photo 1A). Opaque white at first, but shortly before hatching turns white and transparent, showing the developed first larval stage and still wrapped by corium.
Larva
P. sacbriventris has four larval instars. The first instar is creamy-white and semi-transparent, caudate-mandibled, with 13 segments along the body (Photo 1B). These include three thoracic segments, nine abdominals, and a rectangular head capsule strongly sclerotized (0.1497 x 0.1439 mm). Total body length is 0.4406 x 0.1439 mm from the mandibles to the small tail. In the second instar, the head capsule appears to be smaller (0.1121 x 0.1146 mm) in proportion to the body (0.5605 x 0.1146 mm), which is now wider and globular. The body surface remains smooth and 13 body segments are maintained; the apparent tail disappears. The larva is yellowish, more turbid and dark (0.1006 x 0.0478 mm). The third stage is vermiform cylindroids, legless (0.683 x 0.2013 mm) with no visible, strongly sclerotized parts (Photo 1C). Mandibles become almost imperceptible. Body is creamy-white and slightly yellow. The fourth stage has a similar morphology to the third (1.8574 x 0.4635 mm) with large amounts of fat globules that are distributed on the periphery of the posterior capsule (Photo 1D).
Pupa
Pupation occurs within the host puparium. The type of pupa is obtect and 1.9835 x 0.5004 mm in size (Photo 1E). Initially it is completely white, showing only the fat granules; later, the thorax differs from the abdomen and head. More clearly distinguished are the head, antennae, legs, wings, compound eyes, and ocelli—all white as the rest of the body. The cuticle of the pupa gradually darkens to dark brown shortly before adult emergence. The sclerotization process is also gradual, becoming the cuticle hardest and solid over time.
Adult
Adults are black (Photo 1F). Legs are somewhat dingy yellow; hind coxae pale; all the tarsi faintly infuscate. The antenna has 22–23 segments. The female has a simple, smooth, and shiny head. Mesoscutum is highly polished, almost hairless and with no trace of a posterior fovea. Propodeum is evenly convex, smooth and shiny with only the merest trace of sculpture around the spiracle. Mesopleura is highly polished with a short, rugose furrow. Forewings with vein 1-R1 of forewing longer than pterostigma, vein 2-SR of forewing distinctly shorter than vein 3-SR. The ovipositor is projecting slightly beyond the apex of the abdomen. Both male and female are 1.4–1.5 mm long (Photo 1G).
Biology
Parasitism
P. scabriventris is a solitary, koinobiont, and larva-pupal Hymenopteran endoparasitoid of Liriomyza leafminer flies. Females lay their eggs directly inside the second–third stage of Liriomyza larvae bodies. The larvae develop inside the Liriomyza leafminer larvae; however, the parasitized leafminer larvae still consume the tissue of plant leaves until their pupation. P. scabriventris larvae develop through four instars inside the leafminer larva and kill the leafminer in the pupa stage. The mature parasitoid larva then pupates inside the leafminer pupa. The P. scabriventris adult emerges from the leafminer pupa. One adult emerges from a single parasitized pupa. The feeding-stinging phenomenon is observed: larval mortality due to host-feeding-stinging for P. scabriventris is about 12%. Unfertilized females produce only males (arrenotokia parthenogenesis).
Temperature-dependent development
Mean immature developmental period (egg to adult, using L. huidobrensis as the host) decreases with increasing temperature, between 10°C and 30°C, with a mean of 46 and 12 days, respectively (see Annex 7.4.5). The lower theoretical temperature thresholds of eggs, larvae, and pupae development were 5.4°, 3.7°, and 3.5°C, respectively. Mortality of eggs was around 10% at temperatures of 10°–25°C. Larvae mortality was lowest at 25°C (15%). Pupae mortality was lowest between 20°C and 25°C (20%) and increased sharply at temperatures of 10°C (76%) and 30°C (37%). Lifespan decreased with increasing temperature, reaching a maximum of 36 and 22 days at 10ºC and a minimum of 7 days at 30ºC for females and males, respectively. Oviposition of P. scabriventris was significantly affected by temperature, showing a high variability in the progeny production. The lowest oviposition was observed at 10°C, with an average of 36.2 offspring/female and the highest at 15°C, with an average of 151.2 offspring/female. A male:female sex ratio of 1:1 was determined in the offspring of P. scabriventris, with no significant differences between 15°C and 30°C.
The functions established to describe the development time and rate, mortality, and reproduction were compiled into an overall phenology model and the life-table parameters were calculated (see Annex 7.4.5). The intrinsic rate
of increase (r) and the finite rate of increase (λ) had positive values among 9.5°–33°C, indicating a population growth among these temperatures. A peak at 26°C (r: 0.1249; λ: 1.133) was observed. At this temperature, doubling time (Dt) was shortest with 5.5 days. The mean generation time (T) decreased with temperature and was shortest at 31.5°C, with 16 days from egg to egg. The gross reproduction rate and the net (Ro) reproduction rate were highest at 18°C, with 83.5 and 27.5 female offspring/female, respectively. The optimum temperature for overall population growth ranged 24°–27°C. P. scabriventris presents a higher intrinsic and finite rate of increase than L. huidobrensis (section 4.3.1), confirming its potential as an efficient biological control agent of the pest.
Economic impact in pest control
P.scabriventris possesses many qualities that make it a highly potential candidate in classical biocontrol programs for L. huidobrensis. This includes its wide range of geographical and ecological distribution and its significant importance as parasitoid of Agromyzidae flies, which occur as pests in agricultural crops. In the Neotropics, it often is the dominant parasitoid of L. huidobrensis, representing up to 50% of the total parasitism. P. scabriventris is an important parasitoid in the highlands of Peru, accounting for 22.0–41.7% of the total parasitoids reared from L. huidobrensis infesting faba bean (Vicia faba L.), with an average parasitism of 32.6%.
In a classical biological control program, P. scabriventris was imported from Peru and introduced to Kenya and released as an exotic parasitoid. This was done in combination with the pteromalid Halticoptera arduine Walker and the eulophid Chrysocharis flacilla Walker (see sections 5.1.4 and 5.1.6) for controlling the invasive leafminer flies Liriomyza huidobrensis, L. sativae, and L. trifolii (see sections 4.3.1–4.3.3). P. scabriventris was released in 2011 in pilot areas of agro-ecological vegetable production at different altitudes. Field monitoring indicated successful establishment of the parasitoid at all the release sites and a gradual but consistent improvement in total parasitism rate over the years. And while the specific parasitism rates of P. scabriventris is still low, the parasitoid’s spread assessed in December 2013 (just two years after release) reached beyond a 50-km radius at low altitude levels (<1,000 masl) and up to 40 and 30 km radius at high (>1,800 masl) and mid-altitude levels (1,000–1,800 masl).
Geographic distribution
Possible regions of origin: Neotropic: Argentina, Brazil, Chile, and Peru
Introduced and established: In Kenya for the classical biocontrol of Liriomyza huidobrensis, L. sativae, and L. trifolii (Fig. 1).
Potential establishment and efficiency under current and future climates
Changes in global establishment and future distribution
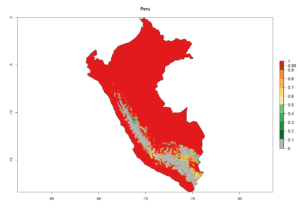
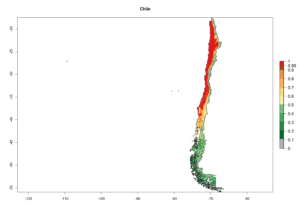
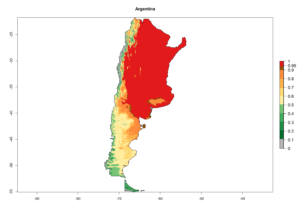
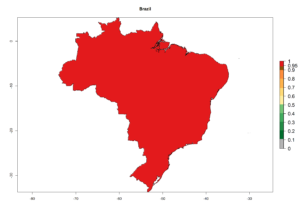
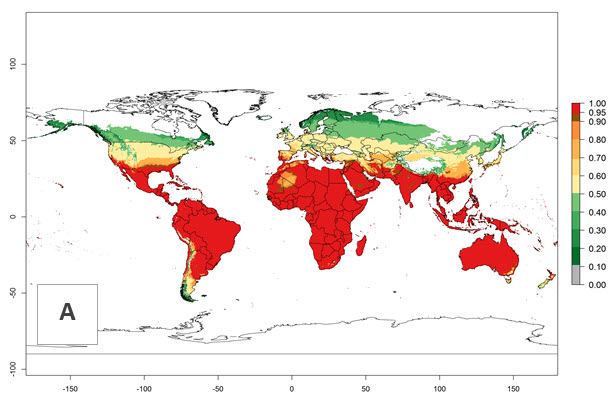
A high establishment index (EI>0.95) of P. scabriventris is predicted in the regions of its origin as in central Argentina (Cordoba, 800 masl); central highlands of Peru (3,200–4,042 masl); and in lowland areas of Chile, Peru, and Brazil (Rio Grande do Norte), including areas where P. scabriventris has been introduced as in Kenya (Fig. 2). In zones where the EI drops below the maximum number of 1, the likelihood of long-term establishment is reduced. However, P. scabriventris is already distributed with an EI>0.7–0.9 under open-field conditions in high-elevation regions of South America, as in prepuna (Jujuy) and temperate (Buenos Aires) zones of Argentina.
| Peru | Chile | Argentina |
| Brazil | Kenya |
Figure 2. EI of Phaedrotoma scabriventris in countries where establishment has been reported, according to model predictions for the year 2000. An EI>0.7 indicates regions with potential permanent establishment.
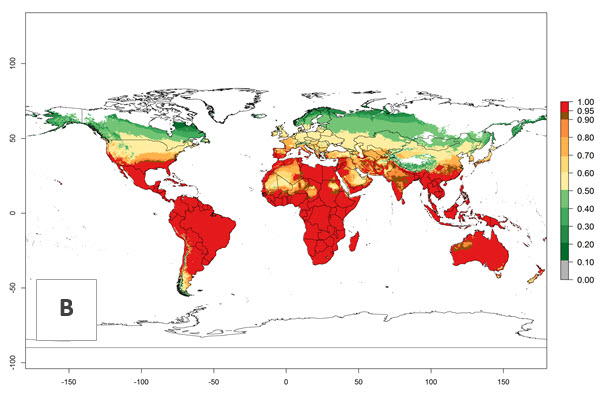
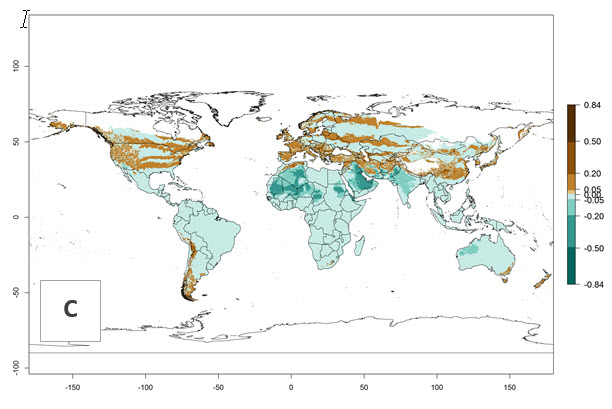
Global predictions for the year 2000 indicate a high potential of establishment of P. scabriventris (EI>0.95) in tropical and most subtropical zones of Africa, America, Asia, and Oceania (Fig. 3A). Here, the leafminer species L. huidobrensis (section 4.3.1), L. sativae (section 4.3.2), and L. trifolii (section 4.3.3) also has a high ERI and a middle establishment potential (EI>0.7) in European Mediterranean countries and central China. Global predictions for the year 2050 indicate a slight decrease in the establishment potential (<-0.05) of P. scabriventris in most of the tropical and subtropical zones; however, the parasitoid will continue to have a high potential of establishment (EI>0.95) and for successful naturalization in biocontrol programs of Liriomyza sp. in many countries and vegetable-growing regions (Fig. 3B, C). A slight range expansion is projected to some temperate regions of southern South America, Europe (Mediterranean zones), Asia (central China), and North America (southern USA) with an EI>0.7–0.9.
 |
 |
 |
|
Figure 3. Changes in establishment and potential distribution worldwide of Phaedrotoma scabriventris according to model predictions, using the EI for the years 2000 (A), and 2050 (B), and changes of the EI between 2000 and 2050 (C). An EI>0.7 indicates regions with potential permanent establishment.
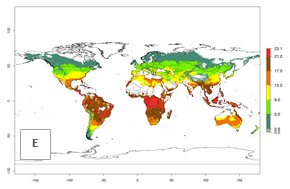
Changes in global abundance
The generation index (GI) for the year 2000 estimates a mean number of >13 generations per year for tropical regions in America, Africa, Asia, and Australia and of 9–13 generations per year in subtropical regions (Fig. 3A). The number of generations estimated in countries where P. scabriventris is established today ranges 16–21 per year in Brazil (Rio Grande do Norte); 9–13 per year in central Argentina (Cordoba) and coast of Peru; 6–9 per year at the coast of Chile, in the highlands of Peru, and in eastern Argentina (Buenos Aires); and 3–6 per year in the prepuna of Argentina (Jujuy). The GI change indicates a potential increase of 1–4 generations per year can be expected in most regions of the world due to climate change until the year 2050 (Fig. 3B, C).
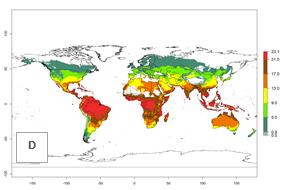
Global maps of the activity index (AI) in year 2000 estimates a high activity (AI>17) of P. scabriventris in tropical regions of Central and South America, the Caribbean, Central and East Africa, and Southeast Asia (Fig. 3D). The potential population growth in countries where P. scabriventris is established today ranges AI>21–23 in Brazil (Rio Grande do Norte), AI>13–17 in central Argentina (Cordoba), AI>5–9 in Peruvian highlands, and AI>0.9–5 in the prepuna of Argentina (Jujuy). Predictions of changes for the 2050 temperature scenario estimate an increase in the potential growth of P. scabriventris by a factor of 1–5 in most subtropical and temperate regions. A decrease by a factor of up to -5 is estimated for tropical regions.
| GI | AI | |
| 2000 | 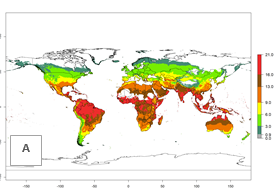 |
 |
| 2050 | 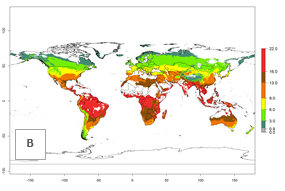 |
 |
| Index change (2000–2050) | 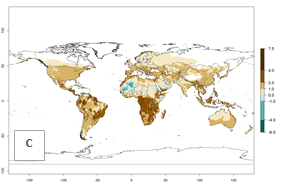 |
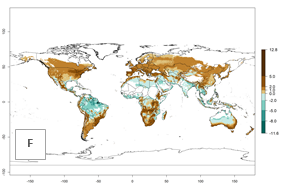 |
Figure 4. Changes in abundance (GI, number of generations/year) and activity (AI, potential population growth) of Phaedrotoma scabriventris in production systems worldwide according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
Changes in regional establishment and distribution in Africa
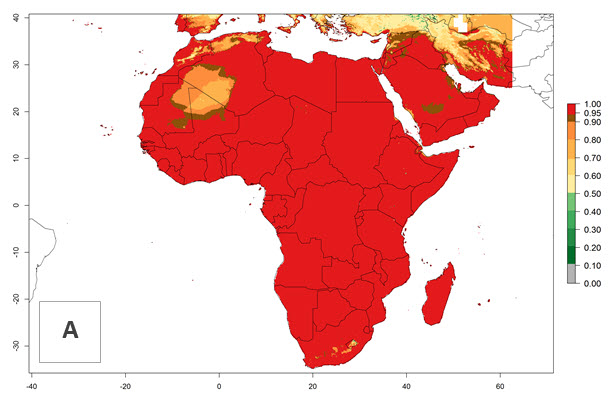
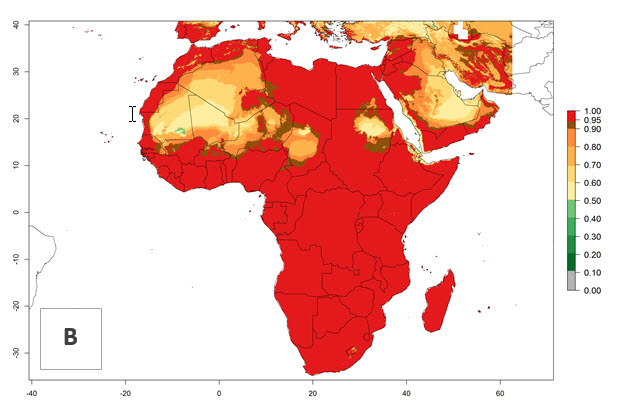
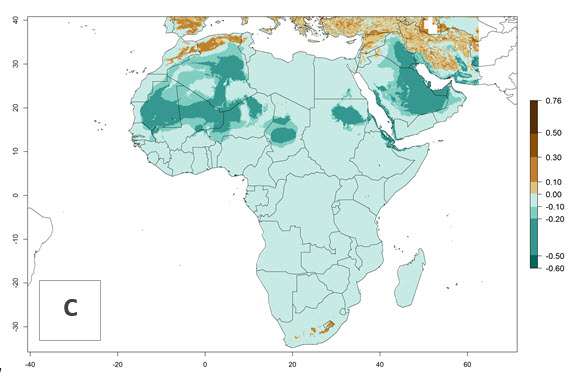
P. scabriventris was introduced into areas of Kenya with a high establishment index (EI>0.95), with subsequent successful establishment. According to the mapping results, a successful establishment (EI>0.95) could also be expected in most countries of North, West, East, Central, and Southern Africa under the year 2000 temperature conditions (Fig. 5A). Owing to climate change, P. scabriventris will maintain a high establishment potential (EI>0.95) in the year 2050 in most regions of West, East, Southern, and Central Africa, as well as in the Mediterranean region of North African countries, where only a slight decrease in establishment (<-0.05) is predicted (Fig. 5B, C). As the Sahara region will not represent favorable future temperature conditions for Liriomyza spp., it will also become more unsuitable for P. scabriventris.
 |
 |
 |
|
Figure 5. Changes in establishment and potential distribution of Phaedrotoma scabriventris in Africa according to model predictions, using the EI for the years 2000 (A) and 2050 (B) and changes of the EI between 2000 and 2050 (C). An EI>0.7 indicates regions with permanent establishment.
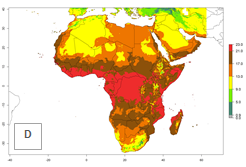
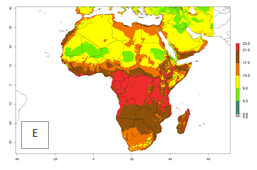
Changes in abundance
The GI for the year 2000 estimates 13–19 generations per year for tropical zones of Africa and 9–16 generations per year for most subtropical zones (Fig. 6A). For the Mediterranean region of Morocco, Algeria, and Tunisia; temperate zones of South Africa; and highland countries of East Africa, 6–9 generations per year are predicted. Predictions for the year 2050 scenario indicate an increase of 1–3 generations per year (Fig. 6B, C) in sub-Saharan Africa and the Mediterranean region. The GI is strongly correlated with the AI, and the activity of P. scabriventris is highest in tropical countries (AI>17). For the 2050 scenario an increase (up to 5) is expected for sub-Saharan Africa countries and the Mediterranean region (Fig. 6E, F).
| GI | AI | |
| 2000 | 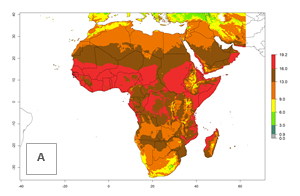 |
 |
| 2050 | 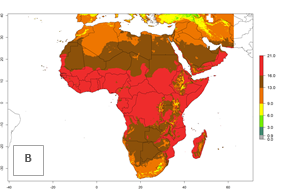 |
 |
| Index change (2000 – 2050) | 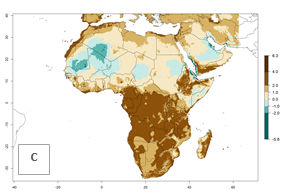 |
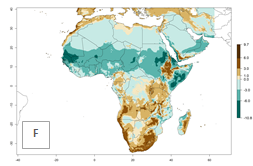 |
Figure 6. Changes in abundance (GI, number of generations/year) and activity (AI, potential population growth) of Phaedrotoma scabriventris in Africa according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
Potential release areas in Africa
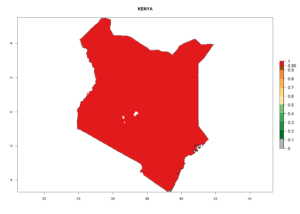
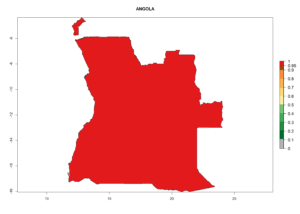
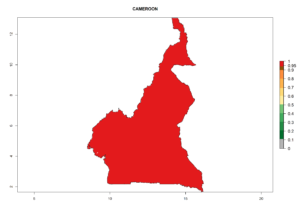
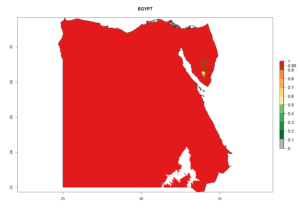
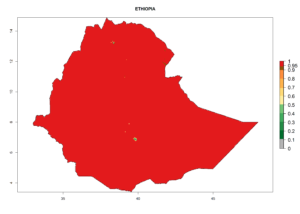
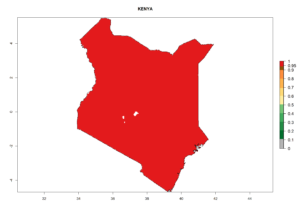
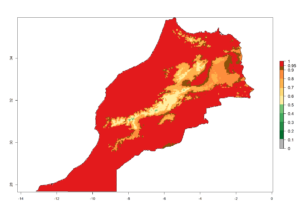
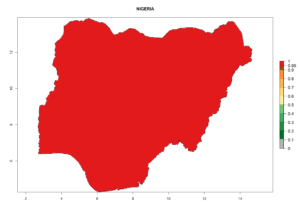
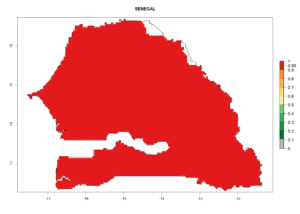
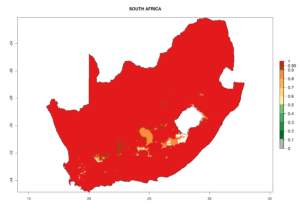
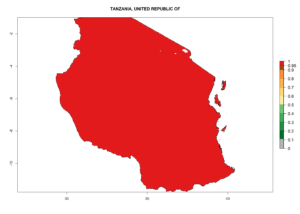
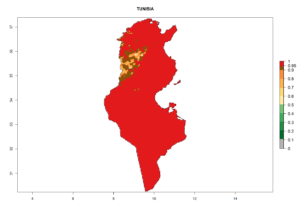
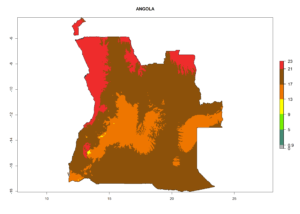
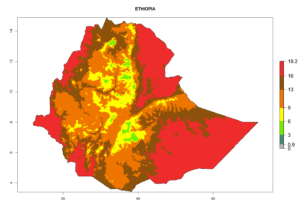
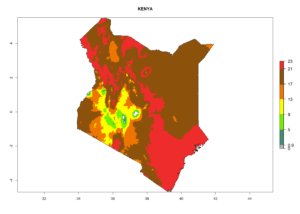
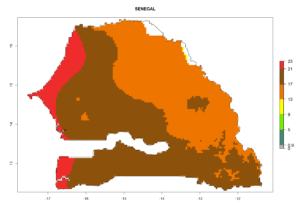
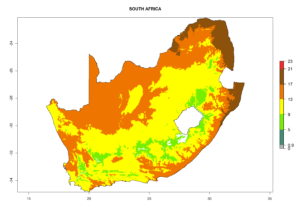
Considering the highest ERI, GI, and AI of Liriomyza species in areas in Africa (section 4.3.1), potential countries to release P. scabriventris under the present climate are mainly in East (Ethiopia, Kenya, Uganda, Rwanda, Tanzania, Madagascar), Central (Angola), and West (Senegal, Nigeria, Cameroon) Africa. In these countries the likelihood of establishment of P. scabriventris is expected to be very high (EI>0.95) and associated with a GI>9 (i.e., more than 9 generations per year) and an AI>13 (Fig. 7). P. scabriventris could also be considered for releases in the Mediterranean region (Morocco, Tunisia, Egypt) and in countries in Southern Africa (South Africa) due to high EI (EI>0.95) but with a middle GI (6–9 generations per year) and an AI (9–13).
| EI | GI | AI |
| a) Angola | 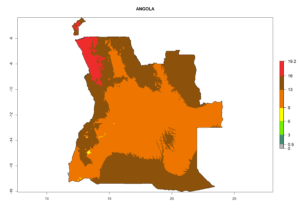 |
 |
| b) Cameroon | 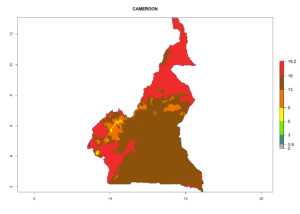 |
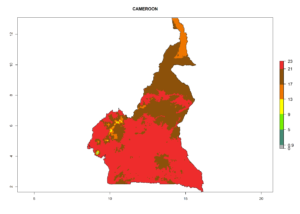 |
| c) Egypt | 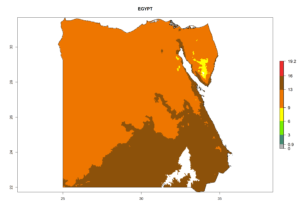 |
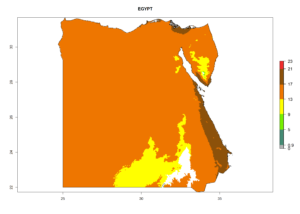 |
| d) Ethiopia | 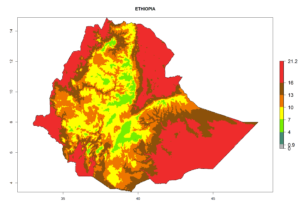 |
 |
| e) Kenya | 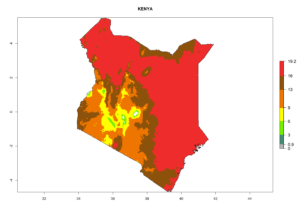 |
 |
| f) Morocco | 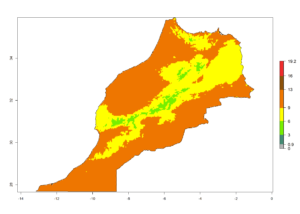 |
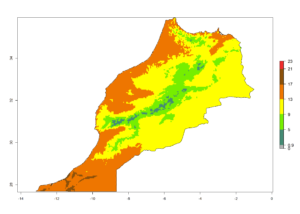 |
| g) Nigeria | 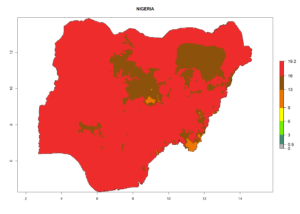 |
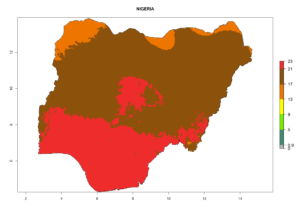 |
| h) Senegal | 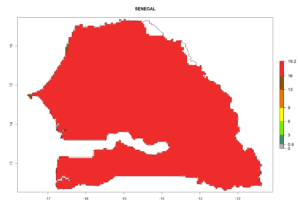 |
 |
| i) South Africa | 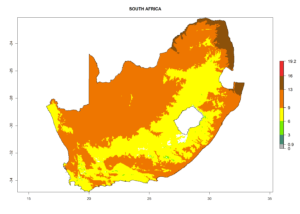 |
 |
| j) Tanzania | 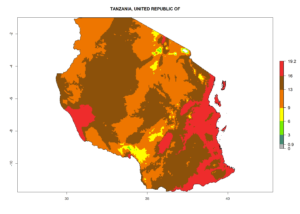 |
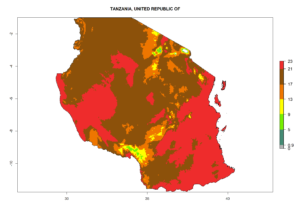 |
| k) Tunisia | 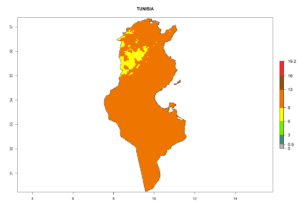 |
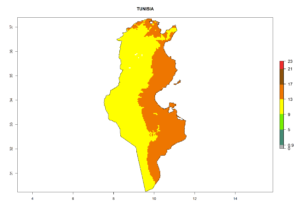 |
| l) Uganda | 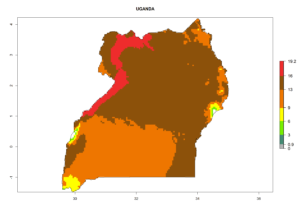 |
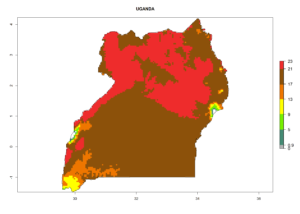 |
Figure 7. EI, abundance (GI, number of generations/year), and activity (AI, potential population growth) of Phaedrotoma scabriventris in selected African countries according to model predictions for the year 2000. An EI>0.7 indicates regions with permanent establishment.
Risks to non-targets
No risks are reported. P. scabriventris is a parasitoid of Agromyzidae leafminer flies with a wide host range. This also includes species that are non-agricultural pests. In the region of evolution the parasitoid lives in a natural balance and equilibrium with any kind of leafminer host, where (to our knowledge) it has not caused the extinction of any species. After its release in new agro-ecosystems, it is hoped that the species will adapt and naturalize in its new environment in order to achieve high impact by reducing the infestation of agricultural Agromyzidae pests like L. huidobrensis. In addition to parasitism of target pests, the introduced parasitoid may also parasitize local leafminer species occurring in the natural environment. This, however, is unlikely to cause an extinction of a species.
Further reading
Campos, T., A. Takematsu, S. Rodríguez, E. Bitran, D. Oliveira, and S. Chiba. 1984. Controle quimico do diptero minador Liriomyza sp. em feijoeiro (Phaseolus vulgaris L.). Biologico 50(2): 33–38.
Costa-Lima, T.C. 2011. Bioecologia y competencia de dos especies de parasitoides neotropicales (Hymenoptera: Braconidae y Eulophidae) de Liriomyza sativae Blanchard, 1938 (Diptera: Agromyzidae). PhD diss., Universidad de Sao Paulo, Sao Paulo, Brazil.
Fischer, M. 1977. Hymenoptera, Braconidae (Opiinae II-Amerika). Das Tierreich 96: 1–1001.
Muchemi, S.K., Ngichop, F.C., and K.M. Fiaboe. 2014. Report on the establishment of the released hymenoptera Phaedrotoma scabriventris Nixon (Braconidae), Halticoptera arduine (Walker) (Pteromalidae) and Chrysocharis flacilla (Walker) (Eulophidae) in vegetable production systems of Kenya. International Centre of Insect Physiology and Ecology (icipe), Nairobi, Kenya, 13 pp.
Mujica, N., and F. Cisneros. 1997. Developing IPM components for Leafminer Fly in the Cañete Valley of Peru. In: International Potato Center Program Report 1995-96. International Potato Center, Lima, Peru, 177–184.
Mujica, N., and J. Kroschel. 2011. Leafminer Fly (Diptera: Agromyzidae) Occurrence, Distribution, and Parasitoid Associations in Field and Vegetable Crops along the Peruvian Coast. Environmental Entomology 40(2): 217–230.
Neder de Roman, L., and M. Arce de Hamity. 1984. Review of and new contributions to knowledge of the bioecology of Liriomyza huidobrensis (Diptera: Agromyzidae). Acta Zoologica Lilloana 37(2): 295–301.
Nixon, G. 1955. Los Insectos de las Islas Juan Fernández. Revista Chilena de Entomología 4: 159–165.
Salvo, A., and G. Valladares. 1995: Complejo parasítico (Hymenoptera: Parasitica) de Liriomyza huidobrensis (Diptera: Agromyzidae) en haba. Agriscientia 12: 39–47.
Salvo, A., M. Fenoglio, and M. Videla. 2005. Parasitism of a leafminer in managed versus natural habitats. Agriculture, Ecosystems and Environment 109(3/4): 213–220.
Sanchez, V.G., and I. Redolfi de Huiza. 1985. Parasitoids of Liriomyza huidobrensis and Scrobipalpula absoluta in potatoes cultivated in Lima (Peru). Revista Peruana de Entomología 28: 81–84.
Valencia, C. 2008. Efecto de la temperatura sobre el desarrollo, longevidad y fecundidad de Phaedrotoma scabriventris (Nixon) (Hym.: Braconidae) parasitoide de Liriomyza huidobrensis (Blanchard) (Dip.: Agromyzidae). BSc thesis, Universidad Nacional Agraria La Molina, Lima, Peru.
Valladares, G., and A. Salvo. 2001. Community dynamics of leafminers (Diptera: Agromyzidae) and their parasitoids (Hymenoptera) in a natural habitat from Central Argentina. Acta oecologica 22: 301–309.
Van Achterberg, C., and A. Salvo. 1997. Reared Opiinae (Hymenoptera: Braconidae) from Argentina. Zoologische Mededelingen (Leiden) 71: 189–214.