4.4.1 Maize Pests / Spotted stemborer, Chilo partellus (Swinhoe 1885)![]()
Synonyms: Argyria lutulentalis (Tams 1932)
Chilo zonellus (Swinhoe 1884)
Crambus zonellus (Swinhoe 1884)
Taxonomic position: Insecta, Lepidoptera, Crambidae
Authors: G. Ong’amo, N. Khadioli, B. Le Ru, N. Mujica, & P. Carhuapoma
Common names
Spotted stalkborer, pink borer, spotted sorghum stemborer (English), pyrale tachée de la tige du sorgho (French), blassroter Stengelbohrer (German), barrenador manchado del tallo, perforador (Spanish)
Hosts
Chilo partellus exhibits oligophagous feeding behavior on Poaceae family only. Primary host plants infested by C. partellus include maize (Zea mays L.), sorghum [Sorgum bicolor (L.) Moench], finger millet (Eleusine corocana Gaertn.), and pearl millet [Pennisetum glaucum (L.) R. Br.]. Secondary host plants include rice (Oryza sativa L.), sugarcane (Saccharum officinarum L.), and fox tail millet [Setaria italica (L.) P. Beauv.]. Wild hosts include many species of wild grasses such as Sorghum arundinaceum (Desv.) Stap, Megathyrsus maximus (Jacq.) B.K. Simon & S.W.L. Jacobs, Pennisetum purpureum Schumach, Arundo donax L., Vossia cuspidata (Roxb.) Griff., Phragmites mauritianus Kunth, Rottboellia cochinchinensis (Lour.) W.D. Clayton, and Echinochloa pyramidalis (Lam.) Hitchc. & Chase.
Detection and identification
Host-plant parts affected are leaves, stems, and seeds. Leaf damage. Neonates produce characteristic leaf windowing while feeding (Photo 1A). They initially feed in the leaf whorl; early larval stages mine in leaves causing yellow streaks (Photo 1B) and after a few days bore down inside the funnel. Feeding on leaf whorl sometime causes “dead heart” (Photo 1C). Seed damage. Seeds can be affected by both internal and external feeding, leading to empty grains and cobs filled with frass (Photo 1D). In sorghum, damage to inflorescences may interfere with grain formation and causes chaffy heads. Stem damage. Larvae may move down outside the stem and then bore into it just above an internode and cause breakage (Photo 1E). Older caterpillars tunnel into stems, eating out extensive galleries, within which they feed and grow for 2 weeks. When larvae are fully grown, they pupate and remain inside the maize stem.

Morphology
Egg
Eggs are laid in overlapping batches on the underside of leaf surfaces near the midrib in 3–5 rows and in groups of 50–100 eggs. The eggs are creamy-white, flattened, ovoid, and about 0.8 mm long (Photo 2A).
Larva
The larvae are creamy-pink to yellowish-brown with groups of dark spots along the back (Photo 2B); the head capsule is reddish-brown. When mature, larvae are about 25 mm long. The larva has a prothoraxic shield, reddish- to dark-brown and shiny. It also has prolegs along the abdomen. Hooks on prolegs are arranged in a complete circle.
Pupa
Pupae are 15 mm long. They are slender, shiny, and light yellow-brown to dark red-brown (Photo 2C). Pupae bear small spines on dorsal anterior margins of the 5th and 7th abdominal segments. Last abdominal segment has spines. Pupation takes place in a small chamber in the stem.
Adult
Adult moths have a wingspan of 20–30 mm. Forewings are brown-yellowish with darker scale patterns forming longitudinal stripes. The forewings of males are pale-brown (Photo 2D), those of females are much paler (Photo 2E). In males, hindwings are a pale straw color, in females, they are white.

Biology
C. partellus completes 1, 2, or more generations per year, depending on the location and the number of maize-cropping seasons or other hosts available throughout the year. One important aspect is the strategy to diapause, or the “resting period,” relevant to understanding its population dynamics and geographic distribution. In warm low-altitude regions, and with ample crop and grass hosts to maintain larval populations, C. partellus develops continuously throughout the year. The species initiates diapause in the larval stage at higher elevations or during dry seasons. Female moths typically prefer whorl (or “funnel”) stage plants for oviposition. Most eggs in maize are found on lower leaf surfaces, often near the midrib. Upon hatching, early instars move upwards on plants and into the whorl, where they begin feeding on leaf surfaces deep inside the whorl. Mid- to late-instars bore into the midrib or stalk, respectively, or move down the stalk and bore into it just above an internode. In older plants, larvae will sometimes feed on the developing tassel. Mature larvae are about 25 mm long and can be distinguished from Busseola fusca by the hooks on the prolegs (see section 4.4.2). In C. partellus, the hooks are arranged in a complete circle, whereas in B. fusca the hooks are in a crescent. Larvae pupate within the maize stalk, but prior to pupation, the fully grown larva cuts out an exit hole to allow the adult moth to emerge from the plant.
Temperature-dependent development
C. partellus successfully developed from egg to adult between the temperatures of 18ºC and 35ºC, but development failed at 15°C and 38ºC, as no eggs hatched at these temperatures (see Annex 7.3.13). The total mean development time decreased with increasing temperature, from 116.9 days at 18°C to 33.8 days at 30°C, and then increased slightly to 35.5 days at 32ºC and 40.8 days at 35ºC. The percentage mortality of eggs and larvae decreased at temperatures between 18ºC and 30ºC and increased thereafter. The minimum mortality for egg (24.6%) and larvae (10.7%) was observed at 30ºC, and for pupae (0.6%) at 32ºC. The mean longevity significantly decreased with increasing temperature, with 12 and 11 days and 5 and 5.9 days at 18ºC and 35ºC for females and males, respectively. The maximum fecundity of 375.3 eggs was observed at 25ºC.
The models established to describe the development time, survivorship, and reproduction were compiled into an overall stochastic phenology model that allows estimating the C. partellus life-table parameters according to temperature (see Annex 7.3.13). The predicted intrinsic rate of natural increase (rm) indicates that populations develop within the range of 16º–35ºC. The negative r values below 16ºC and above 35ºC indicate that population size is decreasing due to high mortality or very low reproduction. The highest population growth can be expected at 29ºC (rm = 0.0117). Similarly, the finite rate of increase for C. partellus peaked at 29ºC (λ = 1.1237) and was smallest when exposed to 15.5ºC (λ = 0.992) and 35.5ºC (λ = 0.945); λ values of <1 indicate that the population is decreasing. At 29ºC doubling time (Dt) was shortest with 5.9 days. The highest values for reproductive parameters were 23º–24ºC for the gross reproductive rate, with 354.2 female offspring/female. When the mortality of females before the reproduction phase was considered, the net reproductive rate (Ro) was highest at 27º–28ºC, with a mean of 94.8 female offspring/female. The mean generation time (T) decreased with temperature and was shortest at 31.5ºC, with 36.2 days from egg to egg. These simulations indicate that C. partellus is able to establish in all tropical maize production areas of the world.
Means of movement and dispersal
About 55% of newly hatched larvae leave young maize plants on silk threads within 2 hr after emergence. Larvae also walk prior to and after stem penetration. Eggs and actively feeding larvae are transported on growing plants. Translocation of stems, stubble, and other crop residues containing diapausing larvae leads to long-distance dispersal. In addition to translocation of larvae and pupae on stems and stubbles, moths—particularly the males—fly distances in response to females’ pheromone calls, whereas females fly long distances as they search for suitable host plants to lay eggs.
Economic impact
Yield loss estimates vary greatly depending on country, season, maize variety, and soil fertility. In South Africa, yield losses caused by C. partellus in maize and sorghum have exceeded 50%. In some provinces of Mozambique, 100% plant infestation has been recorded resulting in considerable yield losses. Maize yield losses in East Africa attributable to C. partellus infestation are estimated at 15–45%. An extensive field survey in Kenya considering fields with and without insecticidal control revealed that the complex of three stemborer species caused on average annual yield losses of 13.5%, valued at US $80 million/year; losses attributable to C. partellus were estimated at US $23 million/year.
Geographical distribution
C. partellus is found in Asia and in most countries of East and Southern sub-Saharan Africa (SSA) (Fig. 1). C. partellus distribution is highly influenced by altitude and moisture gradients. In Kenya for example, C. partellus populations are most common in dry mid-altitude and dry coastal areas. But the pest also occurs in the moist-transitional and moist mid-altitude (ca. <1,500 masl) agro-ecological zones.
| Asia | Cambodia, India (widespread), Pakistan, Afghanistan, Nepal, Bangladesh, Sri Lanka, Thailand, Laos, Vietnam, Yemen, Israel (restricted), and portions of Indonesia (restricted) |
| Africa | Most countries in East and Southern SSA, including Botswana, Eritrea, Ethiopia, Kenya, Malawi, Mozambique, Somalia, Sudan, South Africa, Lesotho, Swaziland, Tanzania, Uganda, Zambia, Zimbabwe, Comoros Islands; also found in Madagascar |
Phytosanitary risks
The European Plant Protection Organisation (EPPO) code of C. partellus is CHILZO. C. partellus is listed as one of the important crop pests in the CABI Crop Protection Compendium. It is also a pest of significance under surveillance by national plant health institutions in SSA countries that are obligated by the International Plant Protection Convention to discharge plant protection functions. Most of these countries have the Plant Protection Act, which offers guidelines on pest eradication and management programs. Plant parts not known to carry the pest in trade/transport are corms, rhizomes, inflorescences, cones, calyx, roots, and seedlings.
Risk mapping under current and future climates
Global Risks
Changes in establishment and future distribution
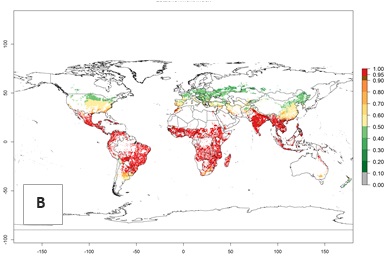
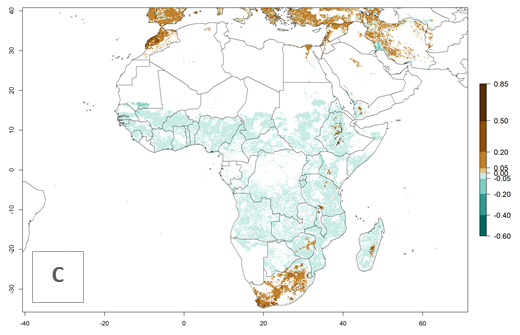
An establishment risk index (ERI)>0.95 indicates that temperature conditions are very suitable to support the establishment of C. partellus, and an ERI<0.95 indicates that the likelihood of long-term establishment is reduced (Fig. 2A). Under the 2000 climate, the ERI>0.95 reflects well the current distribution of C. partellus in countries of East and Southern SSA (Kenya, Tanzania, Uganda, Zambia, Botswana, Eritrea, Ethiopia, Mozambique, Somalia) and Asia (India, Pakistan, Afghanistan, Nepal, Bangladesh, Sri Lanka, Thailand, Laos, Vietnam, Yemen, Israel, Indonesia) (compare with Fig. 1). However, C. partellus has also been reported in countries such as South Africa or Israel, with an ERI>0.7–0.8 in which the pest enters into diapause to survive unsuitable conditions. Further, C. partellus has a great potential of establishment in most parts of West and Central Africa, though it has not been reported in this region. Also, the pest may become established in many parts of Central and South America. The predictions for the year 2050 indicate a potential increase in establishment in subtropical countries of lower latitudes of the Northern (e.g., Egypt, Morocco) and Southern hemispheres (e.g., South Africa, Argentina), including tropical highland areas (Fig. 2B, C).
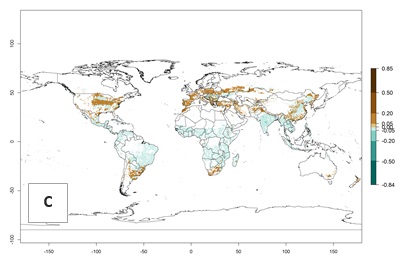
The mean number of generations (potential damage) that might theoretically develop within a year in the year 2000 and the 2050 climate change scenario are visualized in Figure 3A, B. Simulated generation indices (GIs) gave reasonable predictions when compared with generation numbers reported in the literature, with C. partellus reaching up to 10 generations per year in areas with warmer temperatures. Predicted changes in the GI between the years 2000 and 2050 show that all areas in SSA, Southeast Asia, around the Mediterranean Sea, and parts of India will experience an increase of 0.5–2 generations per year. Predictions suggest changes of 0.5–1 generations per year in most parts of West Africa, northern part of East Africa, and lowlands of Southeast Asia. An increase of 1–2 generations per year is expected in all other regions of SSA, around the Mediterranean Sea, in Yemen, and in most highland areas of Southeast Asia and India (Fig. 3C).
The activity index (AI) indicates the potential population growth throughout the year. The AI is predicted to decrease by a factor of 1–2 in many parts of the Sahel in West Africa, northeastern parts of East Africa (Kenya, Somalia), as well as in central India and lowland parts of Southeast Asia. The AI is predicted to increase by a factor of 1–5 in most lowland and mid-altitude parts of Central, East, and Southern Africa; most parts of India; and Southeast Asia (Figs. 3D–F).
| GI | AI | |
| 2000 | 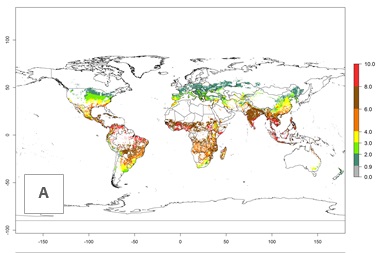 |
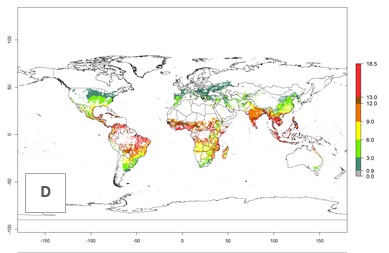 |
| 2050 | 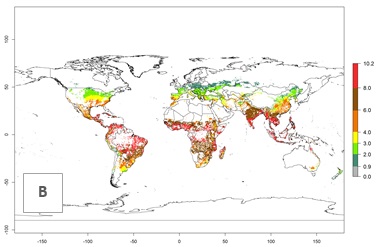 |
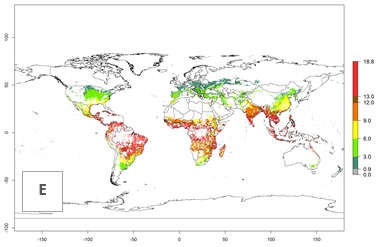 |
| Index change (2000 – 2050) | 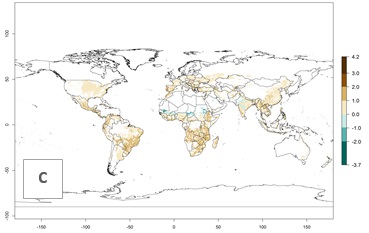 |
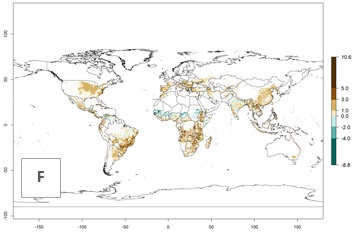 |
Figure 3. Changes in abundance (GI, damage potential) and activity (AI, potential population growth) of the spotted stemborer, Chilo partellus, in maize production systems worldwide according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
Regional Risks for Africa
Changes in establishment and future distribution
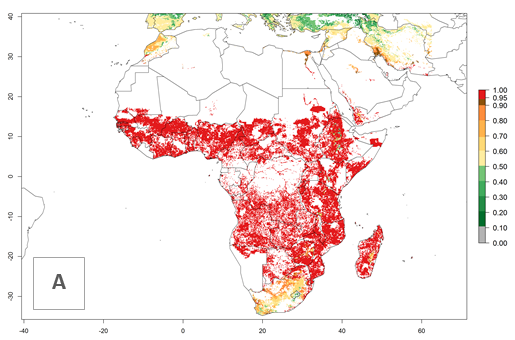
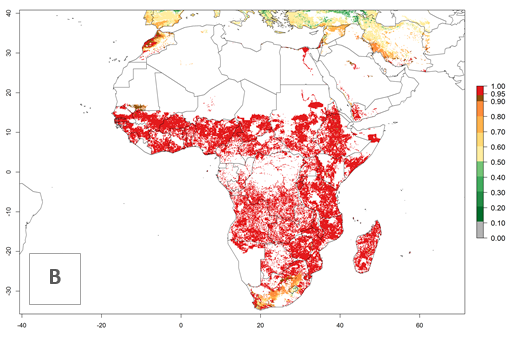
In Africa, C. partellus is already present in countries in East and Southern SSA (e.g., Kenya, Tanzania, Uganda, Zambia, Botswana, Eritrea, Ethiopia, Mozambique, Somalia). C. partellus has a great potential (ERI>0.95) to establish in Central and West Africa (Fig. 4A). The pest is also observed to establish at an ERI>0.7–0.8, as in South Africa under which conditions it enters into diapause. Under the future climate scenario of the year 2050, most maize production areas will remain highly suitable for C. partellus establishment (ERI≥0.95), although a slight reduction is predicted (Fig. 4B, C). The pest can potentially expand its range into highlands of East Africa as well as to subtropical regions of North (e.g., Egypt, Morocco) and Southern Africa.
Figure 4. Changes in establishment and potential distribution of the spotted stemborer, Chilo partellus, in African maize production systems according to model predictions, using the ERI for the years 2000 (A) and 2050 (B), and changes of the ERI between 2000 and 2050 (C). An ERI>0.95 is associated with potential permanent establishment. The pest has been also observed at an ERI.0.7-0.8, in which regions C. partellus enters into diapause to overcome unsuitable conditions.
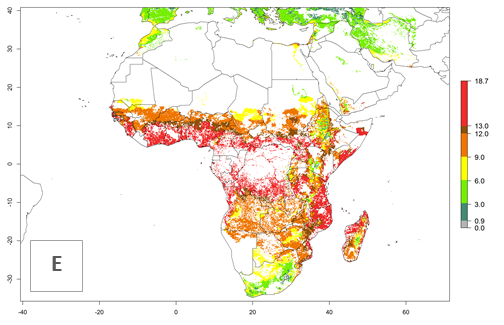
Changes in abundance
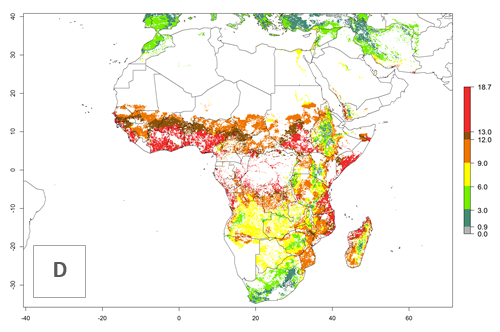
Under current temperature conditions, a GI>4–10 is predicted for C. partellus in African maize production regions (Fig. 5A). For the year 2050, the number of generations will potentially increase by 1–2 per year (Fig. 5B). The GI is highly correlated with the AI (Fig. 5D, E). In the Sahel, temperatures in the year 2050 will exceed those for the pest optimum development and will result in a decrease of the GI and AI (Fig. 5C, F).
| GI | AI | |
| 2000 | 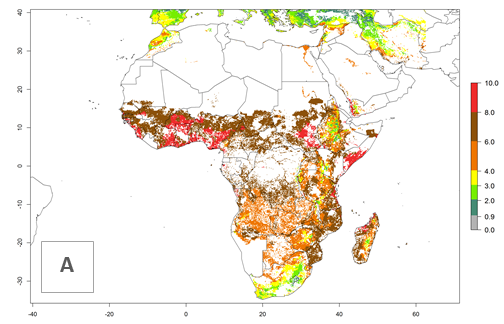 |
 |
| 2050 | 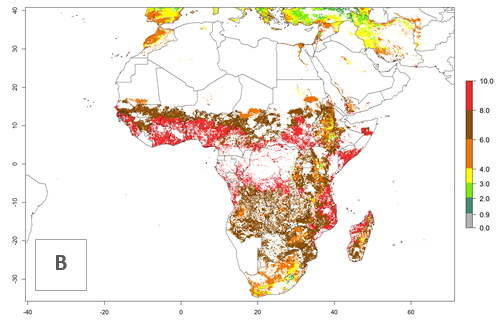 |
 |
| Index change (2000 – 2050) | 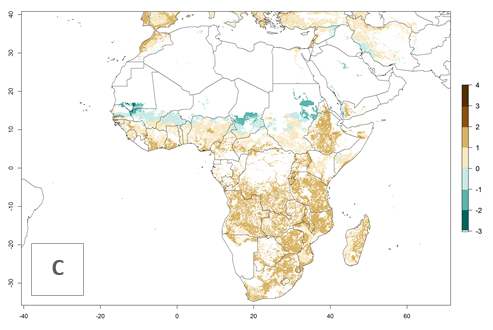 |
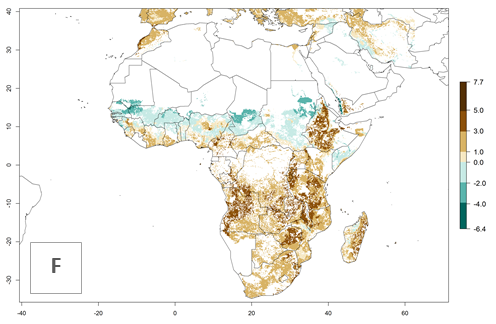 |
Figure 5. Changes in abundance (GI, damage potential) and activity (AI, potential population growth) of the spotted stemborer, Chilo partellus, in African maize production systems according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
Country Risk Maps
Maize is the single most extensively grown crop in Kenya. Establishment of C. partellus is possible in the main maize-growing regions (ERI>0.95), except in small areas of eastern and northwestern Kenya (Fig. 6). C. partellus generations are projected to vary between 2 and 6 generations per year. The highest number of generations (GI>6) is projected for low-altitude areas where temperatures and humidity are currently high. The AI varies from >103 to 1017 for the different maize production regions.
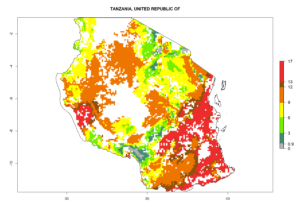
In Tanzania maize is grown in all regions, and an ERI>0.95 is indicated for almost all cultivation areas, except for some small areas in central Tanzania (Fig. 6n). The GI varies 4–8 generations per year for maize-growing regions where the pest is predicted to establish. The AI indicates a potential population growth of 103–1013 per year. For Uganda an ERI>0.95 is indicated for the complete maize cultivation area. The GI predicted 3–6 generations per year and the AI varies from 103 to 1012 per year (Fig. 6o).
| ERI | GI | AI |
a) Angola 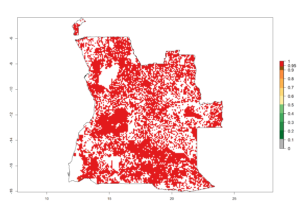 |
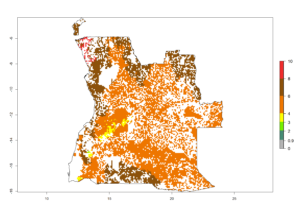 |
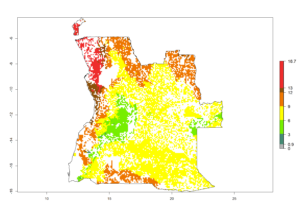 |
b) Burkina Faso 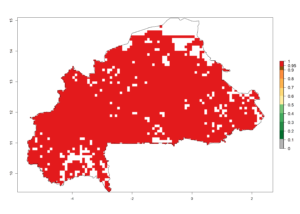 |
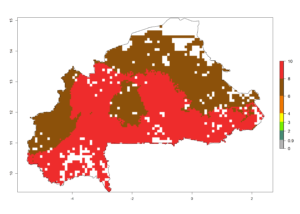 |
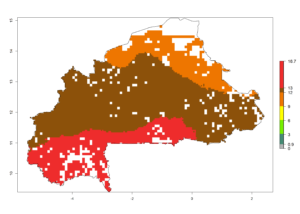 |
c) Burundi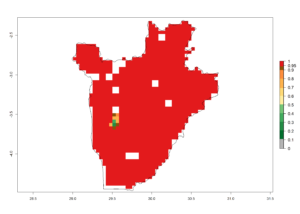 |
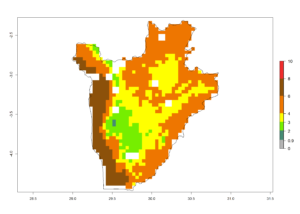 |
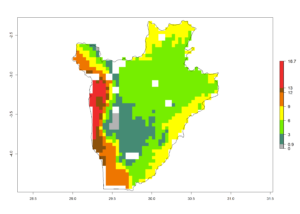 |
d) DR Congo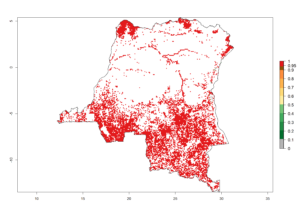 |
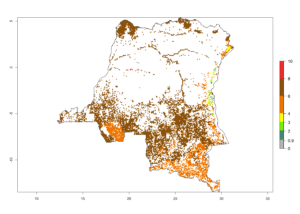 |
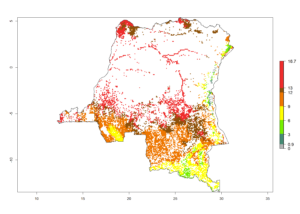 |
e) Kenya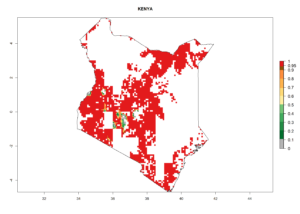 |
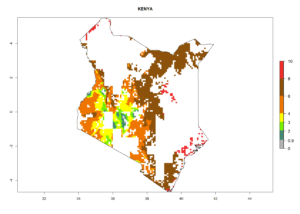 |
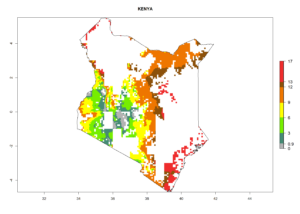 |
| f) Ivory Coast | 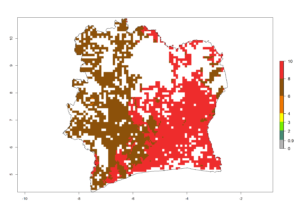 |
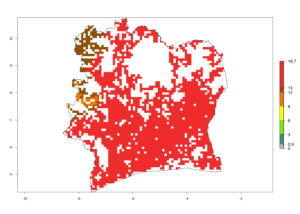 |
g) Madagascar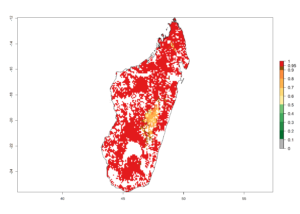 |
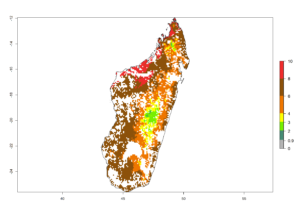 |
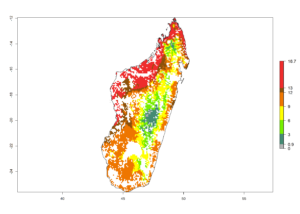 |
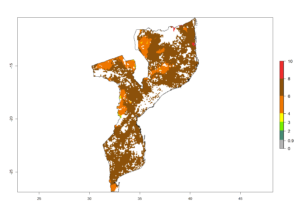
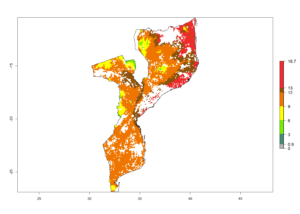
h) Mozambique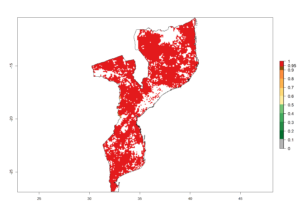 |
 |
 |
i) Nigeria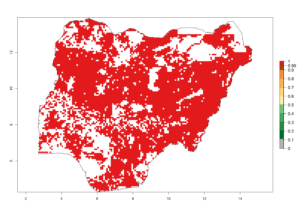 |
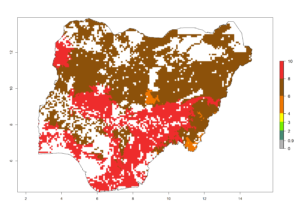 |
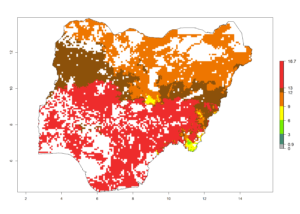 |
j) Rwanda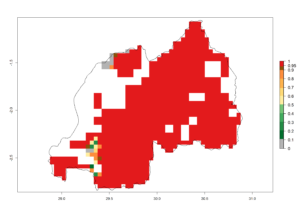 |
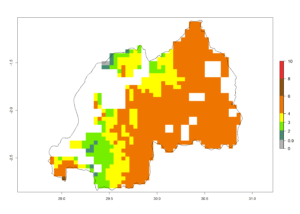 |
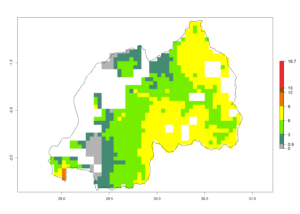 |
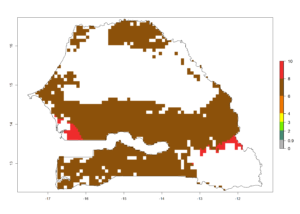
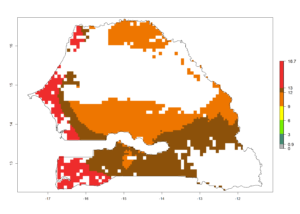
k) Senegal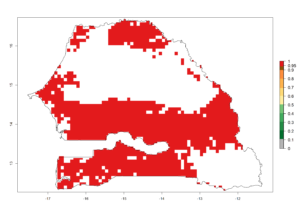 |
 |
 |
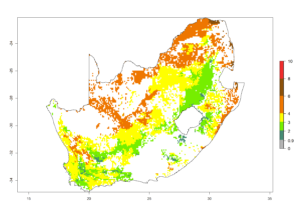
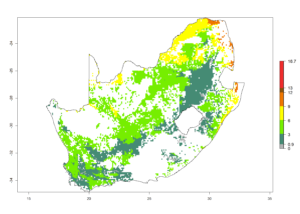
l) South Africa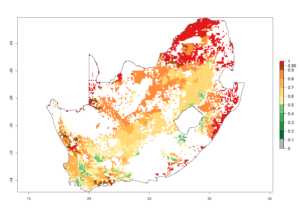 |
 |
 |
m) Sudan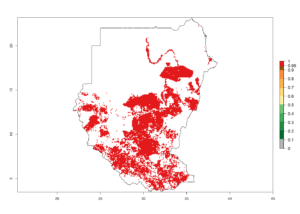 |
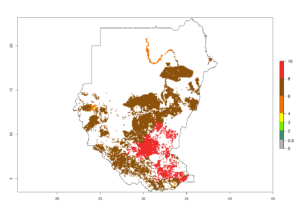 |
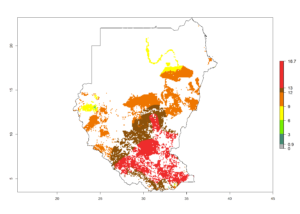 |
n) Tanzania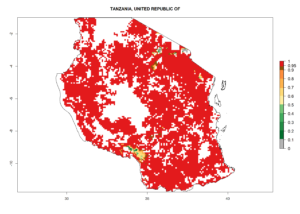 |
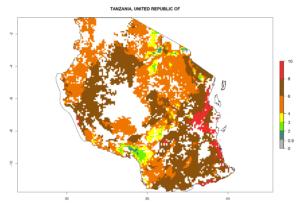 |
 |
| o) Uganda | 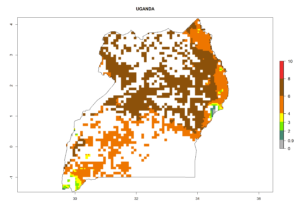 |
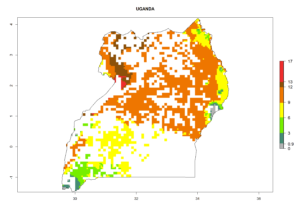 |
Figure 6. ERI, abundance (GI, potential damage), and activity (AI, potential population growth) of the spotted stemborer, Chilo partellus, in maize production systems of selected African countries according to model predictions for the year 2000. An ERI>0.95 is associated with potential permanent establishment.
Phytosanitary measures
The further spread of C. partellus within Africa or from Africa or Asia to other continents and maize production regions might be prevented by controlling the movement of larvae and pupae in stems. To achieve this, plant material should be made subject to movement restrictions. On farms, mechanical destruction of stalks kills larval and pupal stages of the pest. Spreading the stalks out thinly to dry can also kill diapausing larvae.
Adaptation to risk avoidance at farm level
Monitoring pest populations. Pheromone-baited traps are useful devices for monitoring stemborer populations. Sex pheromones of C. partellus have been identified and are available commercially. Trap catches of males can provide useful information for the timing of insecticide applications. More research in trap design and correlations between moth catches and infestations in the field are required before trapping C. partellus can be used to determine economic threshold levels. Additionally, spotted stemborer infestations may be detected by walking through young crops looking for characteristic feeding marks on funnel leaves, the presence of dead hearts, and holes in tunneled stems. Samples of affected stems can be cut open to find caterpillars and pupae. It is best to rear them until they reach the adult stage to identify the exact stemborer species as it is very difficult to identify the species from the larvae and pupae. Spotted stemborers may be detected in older crops and in crop residues by taking random samples and dissect stems to find larvae and pupae.
Biological control. Releases of the parasitic wasps Cotesia flavipes Cameron and Xanthopimpla stemmator Thunberg in classical biocontrol programs resulted in parasitism rates of 30% in East Africa. C. flavipes locates stemborer larvae while they are feeding inside plant stems. The wasp lays about 40 eggs into one stemborer larvae. Upon hatching, the larvae of the parasitic wasp feed internally in the stemborer, after which they exit and spin cocoons. X. stemmator behaves similarly but attacks pupae. Habitat management that conserves parasitoids and predators like ants and earwigs can further improve the biological control of C. partellus.
Cultural control. Practicing good crop hygiene through the destruction of maize residues by burning to get rid of larvae and pupae within the stems and removal of volunteer crop plants and/or alternative hosts prevents carry-over and population build-up. This helps to limit the initial establishment of stemborers that would infest the next crop. Early slashing of maize stubble and laying it out on the ground where the sun’s heat destroys larvae and pupae within can also be done. Manipulation of growing dates, crop rotation, tillage, plant spacing, water management, fertilizer management, field sanitation, mulching, and intercropping further support the management of the pest. Intercropping maize with non-host crops like cassava (Manihot esculenta Crantz) or legumes like cowpea (Vigna unguiculata (L.) Walp.) can reduce spotted stemborer damage. Alternatively, maize can be intercropped with a repellent plant such as silver leaf desmodium (Desmodium uncinatum (Jacq.) DC.) and a trap plant, such as Napier grass (Pennisetum purpureum Schumach.) or molasses grass (Melinis minutiflora P. Beauv.) as a border crop around this intercrop to protect maize from stemborers. The trap plant draws the adult female away from the crop. More eggs are laid on the trap plant than on the crop, but larvae develop poorly or not at all on the trap plant. This practice is known as “push-pull.” Planting maize resistant or tolerant is also a management option for C. partellus.
Chemical control. Chemical control can be achieved by application of granules or dust into the leaf whorl early in crop growth to kill early larval instars. This method has limited effectiveness once the larvae bore into the stem. Neem products (powder from ground neem seeds) are reported being effective and may be applied to the leaf whorl in a 1:1 mixture with dry clay or sawdust. Pesticides are poisons, so it is essential to follow all safety precautions on labels and use only active ingredients that are low in toxicity.
Further reading
Beevor, P.S., H. David, and O.T. Jones. 1990. Female sex pheromones of Chilo spp. (Lepidoptera: Pyralidae) and their development in pest control applications. Insect Science and its Application 11: 787–794.
CABI. 2014a. Chilo partellus (spotted stem borer). Crop Protection Compendium. Available from http://www.cabi.org/isc/datasheet/12859
De Groote, H., W. Overholt, J.O. Ouma, and S. Mugo. 2003. Assessing the potential of Bt maize in Kenya using a GIS model. Paper presented at the International Agricultural Economics Conference, Durban, August 16–22, 2003. Available from http://ageconsearch.umn.edu/bitstream/25854/1/cp03de07.pdf
Den-Yakir, D., M. Chen, S. Sinev, and V. Seplyarsky. 2013. Chilo partellus (Swinhoe) (Lepidoptera; Pyralidae) a new invasive species in Israel. Journal of Applied Entomology 137(5): 398–400.
European Plant Protection Organisation (EPPO). 2011a. Chilo partellus (Swinhoe). Insect pests of cereals in Ethiopia. Available from http://ethiopia.ipm-info.org/chilo-partellus/
———. 2011b. InterAfrican Phytosanitary Council. Available from http://www.au-iapsc.org
———. 2011c. Spotted stemborer. Infonet-Biovision. Available from http://www.infonet-biovision.org/default/ct/92/pests
Kfir, R., W.A. Overholt, Z.R. Khan, and A. Polaszek. 2002. Biology and management of economically important lepidopteran cereal stem borers in Africa. Annual Review of Entomology 47: 701–731.
Khadioli, N., Z.E.H. Tonnang, E. Muchugu, G. Ong’amo, T. Achia, I. Kipchirchir, J. Kroschel, and B. Le Ru. 2014. Effect of temperature on the phenology of Chilo partellus (Swinhoe) (Lepidoptera, Crambidae); simulation of life-table parameters and visualization of spatial pest’s risk in Africa. Bulletin of Entomological Research 104(6): 809–822.
Lucidcentral. 2014. Chilo partellus (Swinhoe 1885) — Spotted Stemborer. Available from http://keys.lucidcentral.org/keys/v3/eafrinet/maize_pests/key/maize_pests/Media/Html/Chilo_partellus_(Swinhoe_1885)_-_Spotted_Stemborer.htm
Overholt, W.A., K.V.N. Maes, and F.R. Goebel. 2001. Field guide to the stemborer larvae of maize, sorghum and sugarcane in Eastern and Southern Africa. Nairobi, Kenya: ICIPE Science Press.
Polaszek, A. 1998. African cereal stemborers; economic importance, taxonomy, natural enemies and control. Wallingford, UK: CAB International.
Seshu Reddy, K.V. 1983. Studies on the stemborer complex in Kenya. Insect Science and its Application 4: 3–10.