4.3.3 Vegetable Pests / American serpentine leafminer, Liriomyza trifolii (Burgess 1880) ![]()
Synonyms: Liriomyza alliovora (Frick)
Agromyza phaseolunata (Frost 1943)
Liriomyza alliivora (Frick 1955)
Liriomyza alliovora (Frick 1955)
Liriomyza phaseolunata (Frost 1943)
Oscinis trifolii (Burgess & Comstock 1880)
Taxonomic position: Insecta, Diptera, Agromyzidae
Authors: N. Mujica, N. Khadioli, B. Le Ru, G. Ong’amo, & P. Carhuapoma
Common names
American serpentine leafminer, chrysanthemum leafminer (English), minador pequeño del frijol (Spanish), Mineuse du gerbera (French), Floridaminierfliege (German)
Hosts
Liriomyza trifolii has been recorded from 29 families with preference for Asteraceae, including Aster spp., Chrysanthemum spp., Gerbera spp., Dahlia spp., Ixeris stolonifera A. Gray, lettuce (Lactuca sativa L.), Lactuca sp., Zinnia sp.; Apiaceae: celery (Apium graveolens L.); Brassicaceae: cabbages (Brassica spp.); Caryophyllaceae: Gypsophila sp.; Chenopodiaceae: spinach (Spinacia oleracea L.), beetroot (Beta vulgaris L.); Cucurbitaceae: cucumbers (Cucumis sp.), pumpkins (Cucurbita spp.); Fabaceae: soybean (Glycine max L.), alfalfa (Medicago sativa L.), bean (Phaseolus vulgaris L.), pea (Pisum sativum L., Pisum spp.), Trifolium sp., faba bean (Vicia faba L.); Liliaceae: onion (Allium cepa L.), garlic (Allium sativum L.); and Solanaceae: peppers (Capsicum annuum L., Capsicum frutescens L.), Petunia spp., potato (Solanum tuberosum L.), and tomato (Lycopersicon esculentum Mill.).
Detection and identification
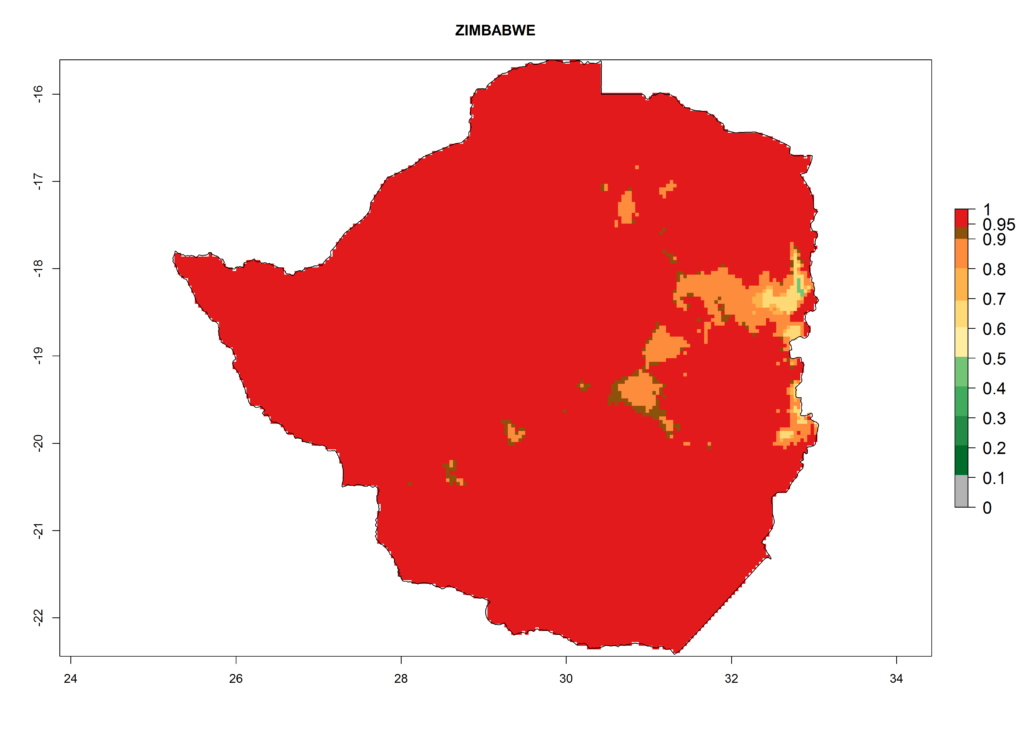
Damage to the plant is caused by the stippling made by the female fly and internal mining by the larvae, allowing pathogenic fungi to enter the leaf via the feeding punctures and mechanical transmission of some plant virus. Both leaf mining and stippling can greatly reduce the level of photosynthesis in the plant, thus affecting plant growth. Punctures caused by females during the feeding and oviposition processes can result in a stippled appearance on foliage, especially at the leaf tip and along the leaf margins (Photo 1D). Feeding punctures appear as white speckles 0.13–0.15 mm in diameter (Photo 1C). Oviposition punctures are smaller (0.05 mm) and are more uniformly round. The major damage is caused by mining of larvae in leaves, which destroys leaf mesophyll. Mines become noticeable about 3–4 days after oviposition and grow larger as the larva matures. Mines in small leaves with limited feeding space are characterized by a secondary blotch (Photo 1A) and are usually white with dampened black and dried brown areas (Photo 1B).

Morphology
Egg
Eggs are oval in shape and small in size, measuring about 0.2–0.3 x 0.10–0.15 mm. Initially they are clear but soon become creamy-white (Photo 2A).
Larva
First-instar larvae are colorless on hatching, turning pale yellow-orange; later instars are yellow-orange (Photo 2B). A headless maggot measures up to 3 mm in length when fully grown. Larvae (and puparia) have a pair of posterior spiracles shaped like a triple cone. Each posterior spiracle opens by three pores, one pore located toward the apex of each cone.
Pupa
The puparium is oval, slightly flattened ventrally, and measures 1.3–2.3 x 0.5–0.75 mm, with variable color, pale yellow-orange often darkening to golden brown (Photo 2C).
Adult
Adults are small flies, measuring less than 2 mm in length. The wings are transparent and measure 1.25–1.9 mm. Scutellum bright-yellow with inner setae usually standing on yellow ground; prescutum and scutum black with gray bloom (Photo 2D). Thorax and abdomen are mostly gray and black, although the ventral surface and legs are yellow. Male and female L. trifolii are generally similar in appearance.
Biology
Female flies puncture the leaves of the host plants, causing lesions that serve as sites for feeding or oviposition. Feeding punctures destroy a larger number of cells and are more clearly visible to the naked eye. Males are unable to puncture leaves but have been observed to feed at punctures produced by females. Eggs are deposited just below the epidermis, on the lower leaf surface of mature leaves found in the middle of the plant. L. trifolii larvae mine the upper surface of the leaves between leaf veins, and mines are normally long, linear, and narrow, although the shape is strongly influenced by the host plant and leaf size. L. trifolii pupates externally, either on the foliage or in the soil just beneath the surface. Pupa is adversely affected by high humidity and drought. On average, females live longer than males.
Temperature-dependent development
L. trifolii completed its development from egg to adult at temperatures of 15º–35ºC (see Annex 7.3.11). Development time decreased with increasing temperature and was shortest with 8.4 days at 35ºC. Mortality was lowest at 25ºC for eggs and larvae (11% for both stages) and at 20ºC for pupae (9%). The highest mortality was observed at 15ºC for eggs (60%) and at 35ºC for larvae (72%) and pupae (67%). Significant differences were observed in the longevity between male and female adults, with female longevity twice as long as males at all temperatures. The lowest senescence rate was observed within the temperature range of 20º–25ºC. Oviposition peaked at 25ºC with 215 eggs per female; at 15º and 35ºC, 12 and 52 eggs were laid on average, respectively.
The established functions (see Annex 7.3.11) were used to estimate the life-table parameters of L. trifolii and to build an overall stochastic phenology model. The intrinsic rate of natural increase (rm) and finite rate of increase (λ) indicate a population growth of 15º–34ºC, with peaks between 24ºC and 28ºC, where a daily population growth of 2.5% was estimated. At this temperature range, doubling time (Dt) was shortest with 2.7 days. The mean generation time (T) decreased with temperature and was shortest at 34ºC with 11.2 days. The gross reproductive rate was highest between 23ºC and 24ºC, with 157 female offspring/female, and net reproductive rate (Ro) was highest at 23ºC, with 58 female offspring/female.
Means of movement and dispersal
Adult flies are capable of limited flight. Dispersal over long distances is on planting material of host species. Cut flowers can also present a risk as a means of dispersal. It should be noted, for example, that the vase life of chrysanthemums is sufficient to allow completion of the life cycle of the pest. L. trifolii are not very active fliers, and in crops showing active mining, the flies may be seen walking rapidly over the leaves with only short jerky flights to adjacent leaves. Expanded shipments of cut flowers appear to be how this species expands its range.
Economic impact
L. trifolii is a major pest of Compositae worldwide—in particular, of chrysanthemums in North America, Colombia, and elsewhere—as well as causing considerable damage in 28 other host families. Extensive mining also causes premature leaf drop, which can result in lack of shading and sun scalding of tomato fruits or reduced tuber filling of potatoes. Presence of unsightly larval mines and adult punctures in the leaf of ornamental plants can further reduce plant value. Leafminers are most damaging when they affect floricultural crops due to low tolerance of such crops to any insect damage. Severely infested leaves may fall, exposing plant stems to wind action, and flower buds and developing fruit to scald, thus reducing crop value. L. trifolii is also known to be a vector of plant viruses. In young plants and seedlings, mining may cause considerable delay in plant development leading to plant loss. L. trifolii is already a serious pest of chrysanthemums in those countries of the European Plant Protection Organisation (EPPO) region where it is established. It is apparently not capable of overwintering outdoors in northern Europe. In Kenya, chrysanthemums were grown commercially before 1976, but L. trifolii was thought to have been introduced in contaminated cuttings from Florida (USA) in 1976, at a large propagating nursery at Masongaleni. And although the nursery was closed by 1979, the establishment of the pest in local wild hosts, together with the dissemination of cuttings from the nursery to other parts of the country as well as abroad, has increased the importance of L. trifolii as a pest in East Africa. It has caused considerable crop losses and loss of overseas markets due to quarantine requirements.
Geographical distribution (permanent establishment)
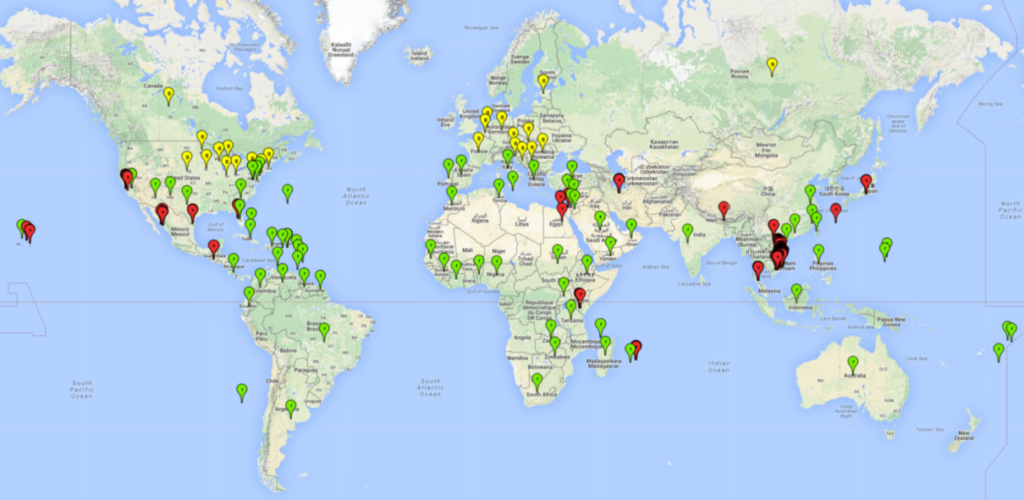
L. trifolii originated in North America and spread to other parts of the world in the 1960–1980s. It has been recorded in numerous countries around the world, presumably associated with the global trade of ornamental plants (Fig. 1).
| Africa | Benin, Egypt, Ethiopia, Guinea, Ivory Coast, Kenya, Madagascar, Mauritius, Mayotte, Morocco, Nigeria, Réunion, Senegal, South Africa, Sudan, Tanzania, Tunisia, Zambia, Zimbabwe |
| Asia | Cambodia, China (Fujian, Guangdong, Hainan, Jiangsu), Cyprus, India (Andhra Pradesh), Iran, Israel, Japan (Honshu, Kyushu), Jordan, Korea Republic, Lebanon, Malaysia, Oman, Yemen, Saudi Arabia, Taiwan, Thailand, Philippines, Turkey, United Arab Emirates, Vietnam |
| Europe | First detected in 1976 in the Netherlands, it is now present in protected crops in Austria, Belgium, Bosnia and Herzegovina, Croatia, Malta, Romania, Serbia, Switzerland, and Turkey; widespread in Cyprus, France, Greece (Crete and Peloponnese), Italy (Sardinia and Sicilia), Portugal (Algarve), and Spain (protected crop, including in Canary Islands). It is eradicated in Bulgaria, Czech Republic, Denmark, Finland, Germany, Hungary, Ireland, Norway, Poland, Sweden, Slovenia, UK |
| North America | Canada (Alberta, Nova Scotia, Ontario, Quebec), USA (outside in New Mexico, Arizona, California, Hawaii, Texas, most eastern states from Florida northward to New Jersey; under glass in other southern states and in Wisconsin, Minnesota, Iowa, Indiana, Ohio) |
| Central America and the Caribbean | Bahamas, Barbados, Bermuda, Costa Rica, Cuba, Dominican Republic, Guadeloupe, Guatemala, Martinique, Mexico (unconfirmed), Netherlands Antilles, Puerto Rico, St. Kitts-Nevis, Trinidad and Tobago |
| South America | Argentina, Brazil (Minas Gerais, Pernambuco, Sao Paulo), Chile, Colombia, Ecuador, French Guiana, Guyana, Venezuela |
| Oceania | American Samoa, Australia (New South Wales, Victoria), Guam, Micronesia, Northern Mariana Islands, Samoa, Tonga |
Phytosanitary risks
L. trifolii is listed as an A2 quarantine pest by the EPPO and is one of the most important recent introductions to the EPPO region. L. trifolii is a major pest of a wide variety of ornamental or vegetable crops grown under glass or as protected crops in the EPPO region. It could also cause damage to these crops grown in the open in warmer parts of the region. Although it is quite widely distributed in the region, there are still several countries where it has not established and others where successful eradication programs have been conducted.
Risks mapping under current and future climates
Global Risks
Changes in establishment and future distribution
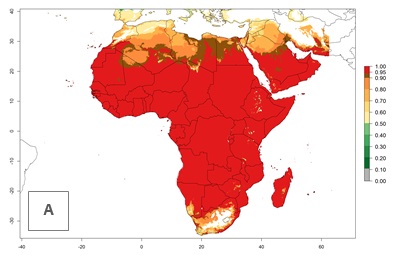
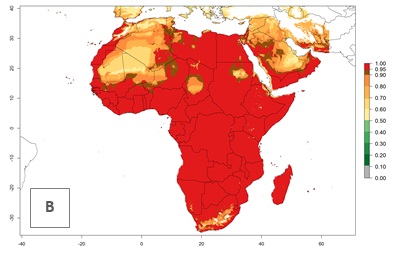
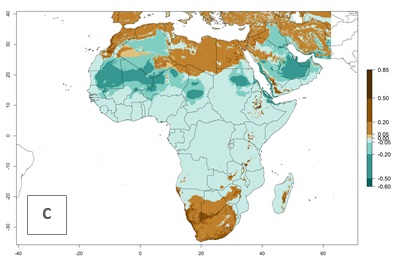
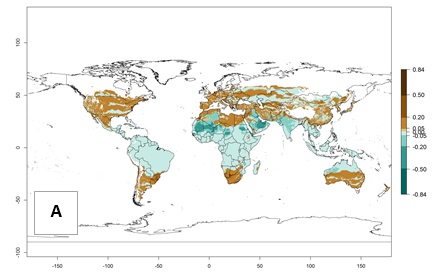
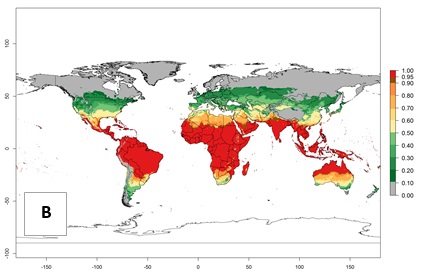
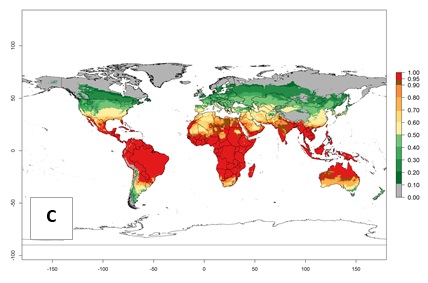
Regions with an establishment risk index (ERI)>0.95 indicate temperature conditions where a certain proportion of L. trifolii population is expected to survive throughout the year (Fig. 2A), and reflects well the current global distribution of the pest under the climate of the year 2000. Those regions include tropical regions of America, Asia, Africa, and Oceania; and in some subtropical areas of Africa (mostly western, central, and eastern countries) and the Middle East (Yemen, Oman, southern Saudi Arabia) (compare with Fig. 1) where the pest is currently widely distributed. L. trifolii also occurs in restricted areas with an ERI>0.7–0.9 (light- and dark-orange zones) as in the U.S. (southern zones of California, Texas, northern Florida); northern areas of Argentina; south of Brazil; northern and southern zones of Africa; southern China (southern Yunnan, Guangxi, Guangdong); and the Middle East (Israel, Lebanon, Jordan, some areas of Saudi Arabia).The lowest occurrence of L. trifolii under open-field conditions with an ERI of 0.6–0.7 is observed in the U.S. (western to southern states from California to North Carolina); central China (Sichuan and Hubei); and Mediterranean European countries (southern areas of Italy such as Sardinia and Sicilia, south of Portugal, Spain). L. trifolii is also reported in regions with a low likelihood of establishment (ERI<0.6) as in temperate regions of northern China; Canada; central and northern U.S. states (Arkansas, Minnesota, Wisconsin, Indiana, Ohio, Iowa, New York); South Korea; Japan; Russia; and European countries corresponding to infestations in greenhouses and not due to survival under open-field conditions. For the year 2050, a non-significant decrease in the risk of L. trifolii establishment is estimated (<-0.05). Therefore the pest will continue to be a high risk in most of the tropical and subtropical areas (ERI>0.95) (Fig. 2B). Owing to climate change, an increase of the ERI between 0.05 and 0.2 is predicted for temperate zones beyond 30° north and south latitude (Fig. 2B, C).
 |
 |
 |
|
Figure 2. Changes in establishment and potential distribution of the American serpentine leafminer fly, Liriomyza trifolii, worldwide according to model predictions, using the ERI for the years 2000 (A) and 2050 (B), and changes of the ERI between 2000 and 2050 (C). An ERI>0.6 is associated with potential permanent establishment.
Changes in abundance
Under the current temperatures, potentially 20–32 generations per year can develop in tropical countries, 12–20 generations per year in most of the subtropical regions, and 3–12 generations per year in temperate zones (Fig. 3A). The generation index (GI) change indicates that in most of the tropical and in some subtropical areas, an increase of 2–5 generations per year is estimated for the 2050 climate change scenario (Fig. 3C). In temperate regions a slight increase of up to 2 generations per year is expected. Globally, simulated GIs for the year 2000 gave reasonable predictions when compared with generation numbers reported in the literature—for example, under tropical conditions, a mean number of 15 generations per year in the Middle East (Israel) and 17 generations per year in Florida have been reported. For the Mediterranean region, 12–20 generations per year were predicted; hence estimations made by the GI are close and consistent with the data reported in the literature.
| GI | AI | |
| 2000 | 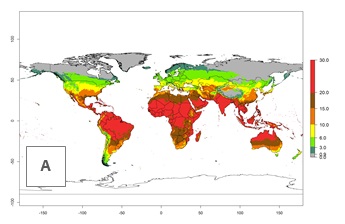 |
 |
| 2050 | 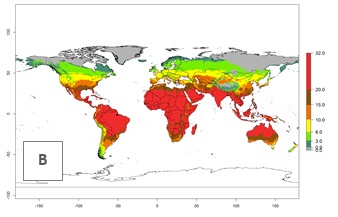 |
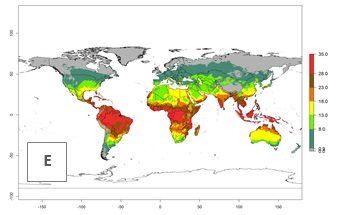 |
| Index change (2000 – 2050) | 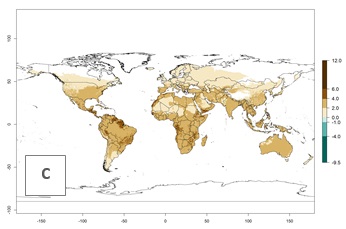 |
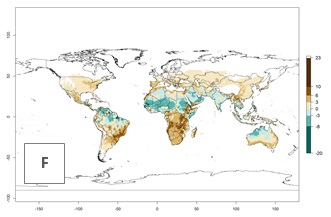 |
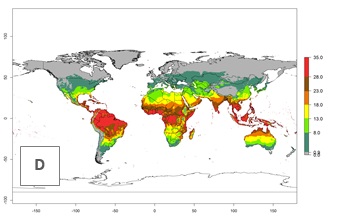
Figure 3. Changes in abundance (GI, damage potential) and activity (activity index [AI], potential population growth) of the American serpentine leafminer fly, Liriomyza trifolii, worldwide according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
Global maps of the activity index (AI) in year 2000 estimate a high activity (AI>35) of L. trifolii in tropical regions of America, Africa, Asia, and Australia (Fig. 3D). A potential population growth of 15–30 is estimated for subtropical regions and a low activity for temperate regions (up to 15). Predictions of changes for the 2050 scenario show a decrease in the activity of L. trifolii (up to -10) in tropical regions, but the pest will continue to have a high activity (AI>35) in most regions. A considerable increase (6–10) is estimated in subtropical zones as in countries in East and Southern Africa, southeast of Brazil, and in highland mountains of South America. The lowest increase in the activity by values up to 6 is predicted in temperate regions (Fig. 3E, F).
Regional Risks for Africa
Changes in establishment and future distribution
L. trifolii is actually present in regions of West (Senegal, Guinea, Ivory Coast, Benin, Nigeria); East (Ethiopia, Kenya, Uganda, Tanzania, Zambia, Mozambique, Madagascar); and North (Sudan) Africa (Fig. 1), with an ERI>0.95 (Fig. 4A).
Figure 4. Changes in establishment and potential distribution of the American serpentine leafminer fly, Liriomyza trifolii, in Africa according to model predictions, using the ERI for the years 2000 (A) and 2050 (B), and changes of the ERI between 2000 and 2050 (C). An ERI>0.6 is associated with potential permanent establishment.
The pest also represents a high risk (ERI>0.95) for most production areas of other countries of West, East, and Central Africa. L. trifolii is established in northern (Morocco, Tunisia, Egypt) and southern (South Africa) regions of Africa, with an ERI<0.9. On the Mediterranean coast of Morocco and Tunisia, as well as in some areas of South Africa, a low establishment potential of an ERI>0.6–0.8 is estimated. Only on the coast of Algeria is an ERI<0.6 shown. Under the year 2050 temperature scenario, a slight decrease (-0.05) in the establishment is predicted, but L. trifolii will remain a potential risk and threat for food production in almost all these African countries. Also, L. trifolii will intensify and expand its potential risk (up to 0.3) in countries of North and Southern Africa (Fig. 4B).
Changes in abundance
The mean number of generations that might theoretically develop within a year in the year 2000 and year 2050 scenario are visualized in Figure 5A, B.
| GI | AI | |
| 2000 | 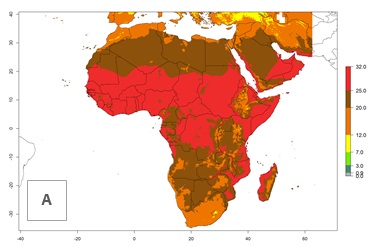 |
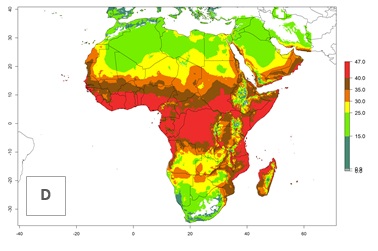 |
| 2050 | 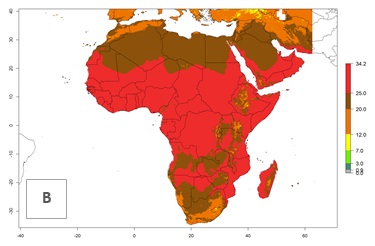 |
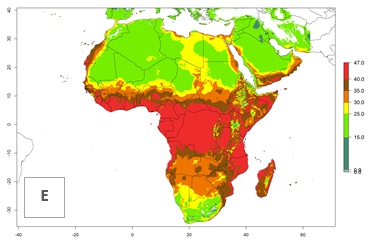 |
| Index change (2000 – 2050) | 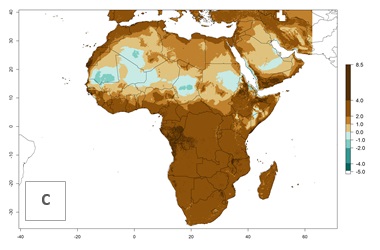 |
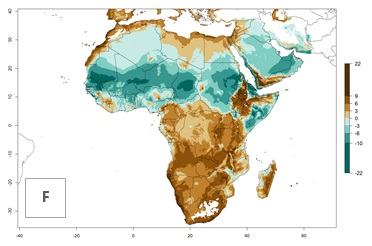 |
Figure 5. Changes in abundance (GI, damage potential) and activity (AI, potential population growth) of the American serpentine leafminer fly, Liriomyza trifolii, in Africa according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
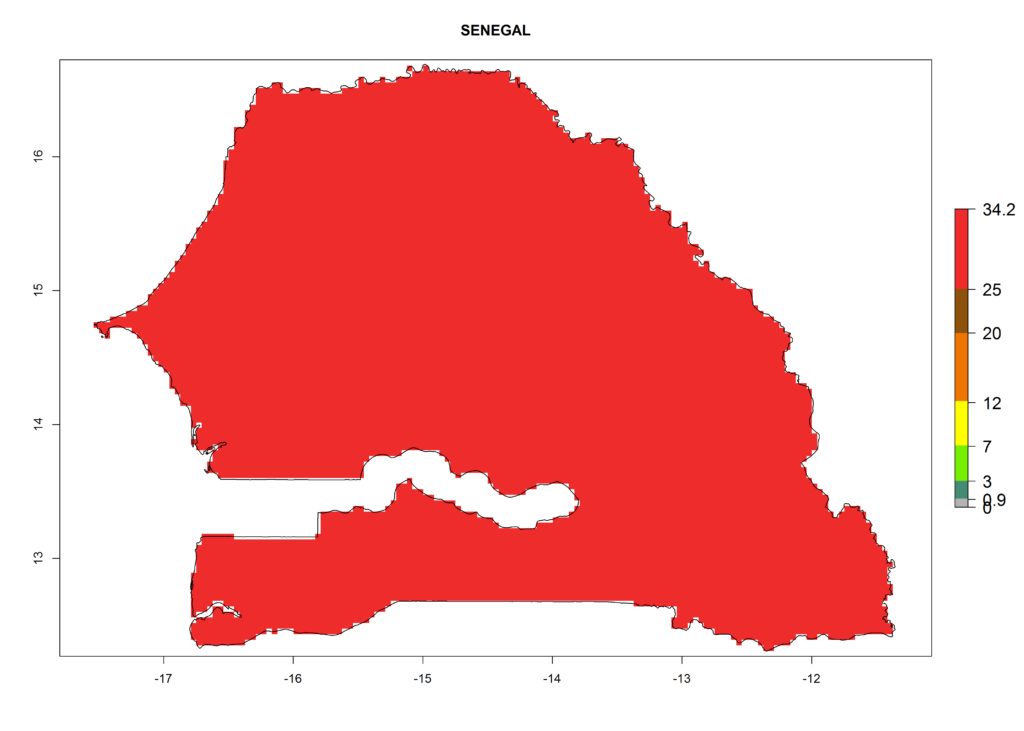
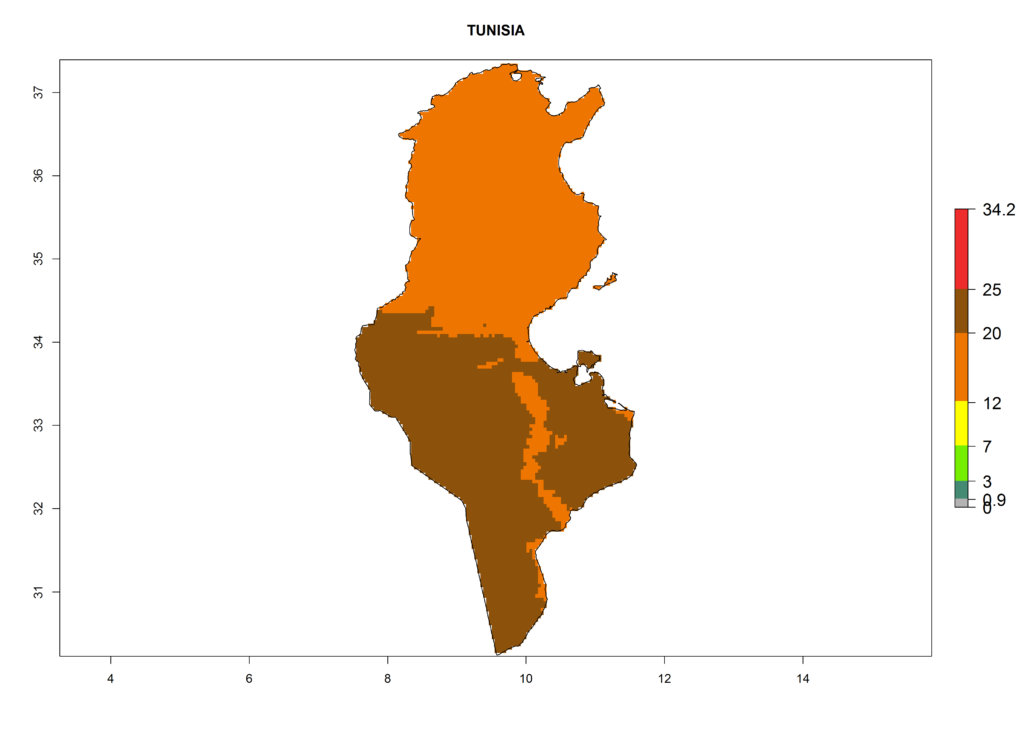
For the year 2000, a high number of generations (GI>25 generations per year) is predicted for tropical African regions, which decrease to 12 generations per year toward the subtropical zones of North (Morocco, northern Algeria) and Southern (South Africa) Africa (Fig. 5A). The GI change for 2050 scenario predicts an increase of 2–4 generations per year for sub-Saharan African countries and the Mediterranean region (Fig. 5B, C). A high increase in the number of generations (4–8.5 per year) is expected for some areas of Central African countries (Angola, Congo, DR Congo, Gabon) and highland areas of East Africa (Ethiopia, Malawi).
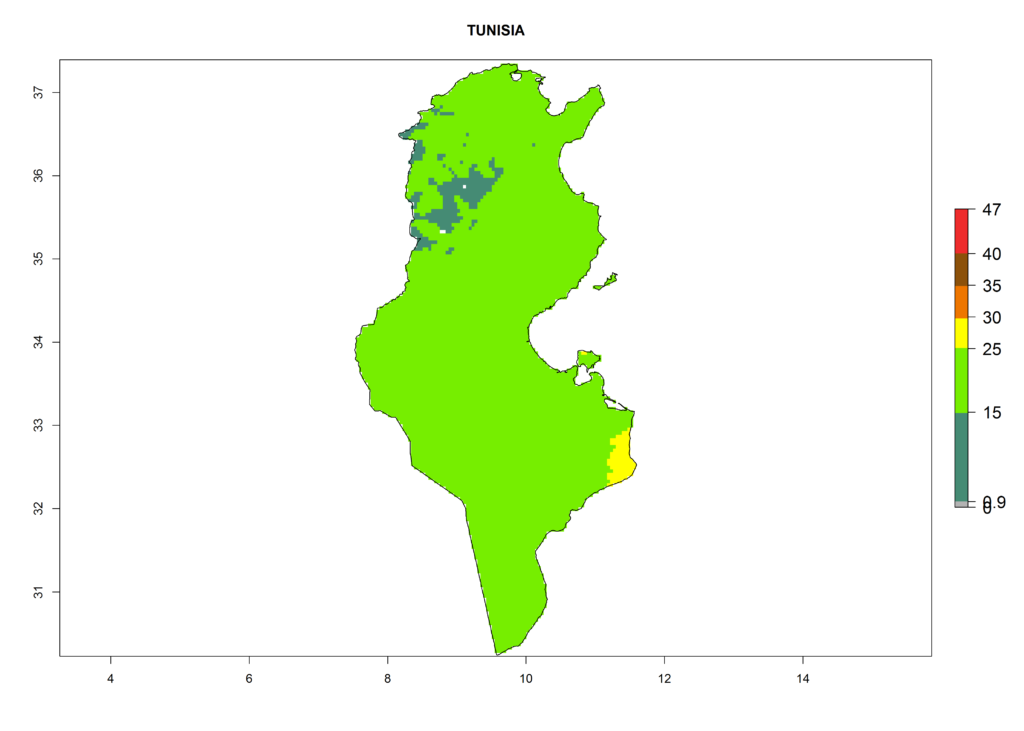
For the year 2000, the highest potential population growth (AI>35) of L. trifolii is predicted for tropical regions of Africa. The activity decreases toward the north and south of the continent (AI<18) (Fig. 5D). Owing to climate change, it is estimated that in Central and Southern Africa, as well in the Mediterranean region, the activity will further increase between the year 2000 and 2050 as demonstrated with the AI change (Fig. 5E, F).
Country Risk Maps
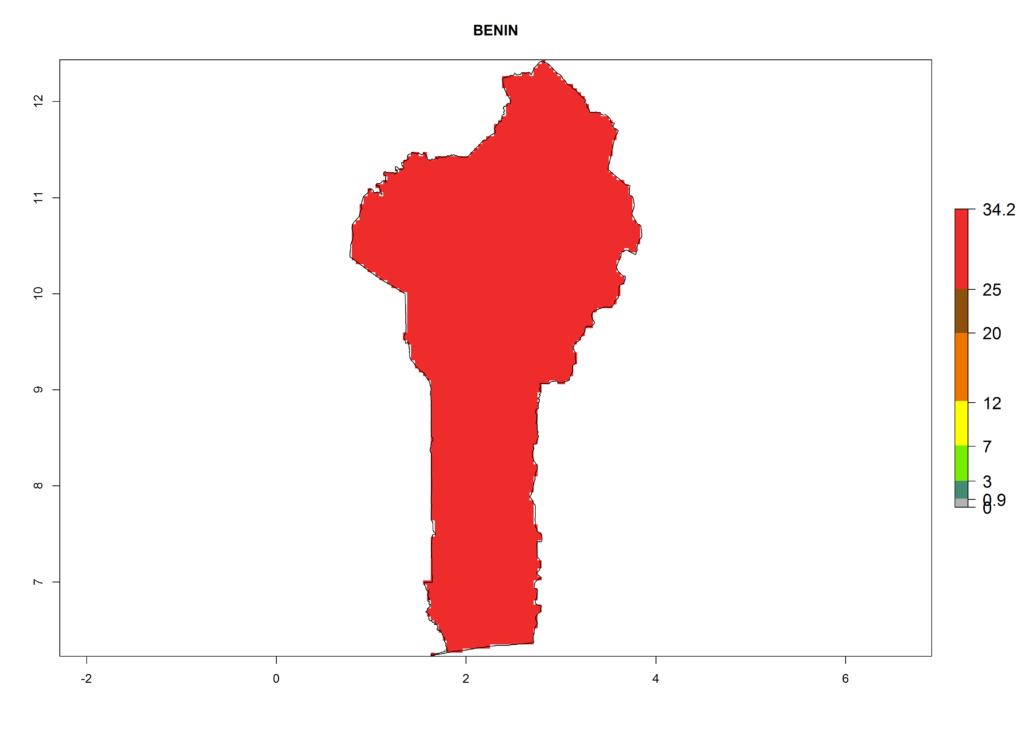
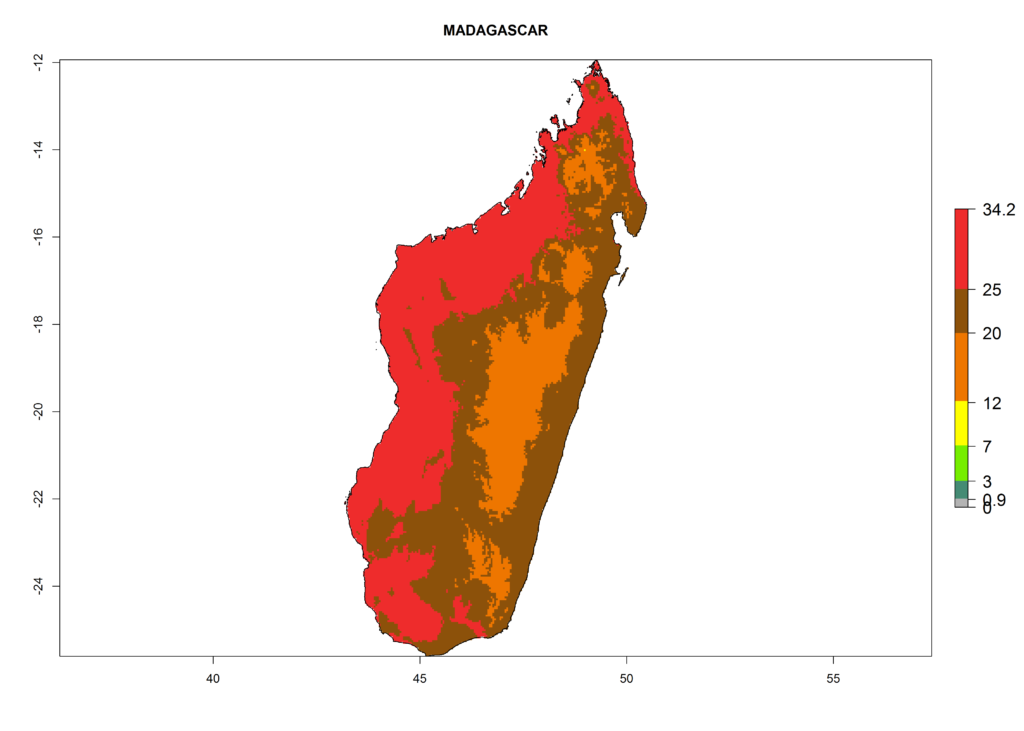
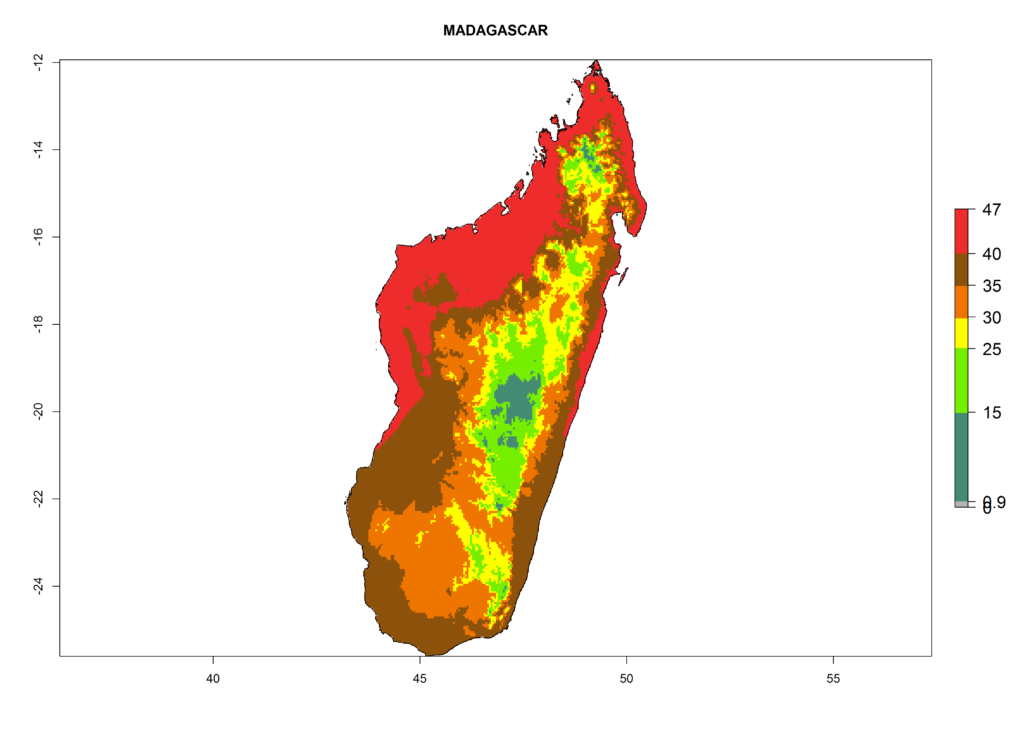
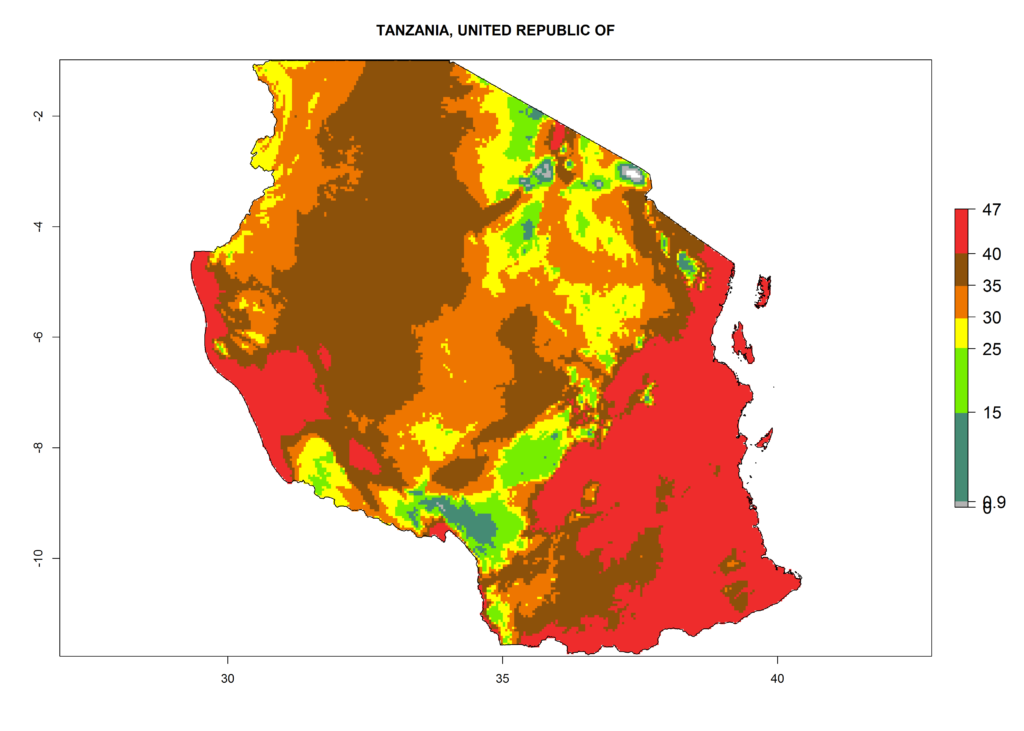
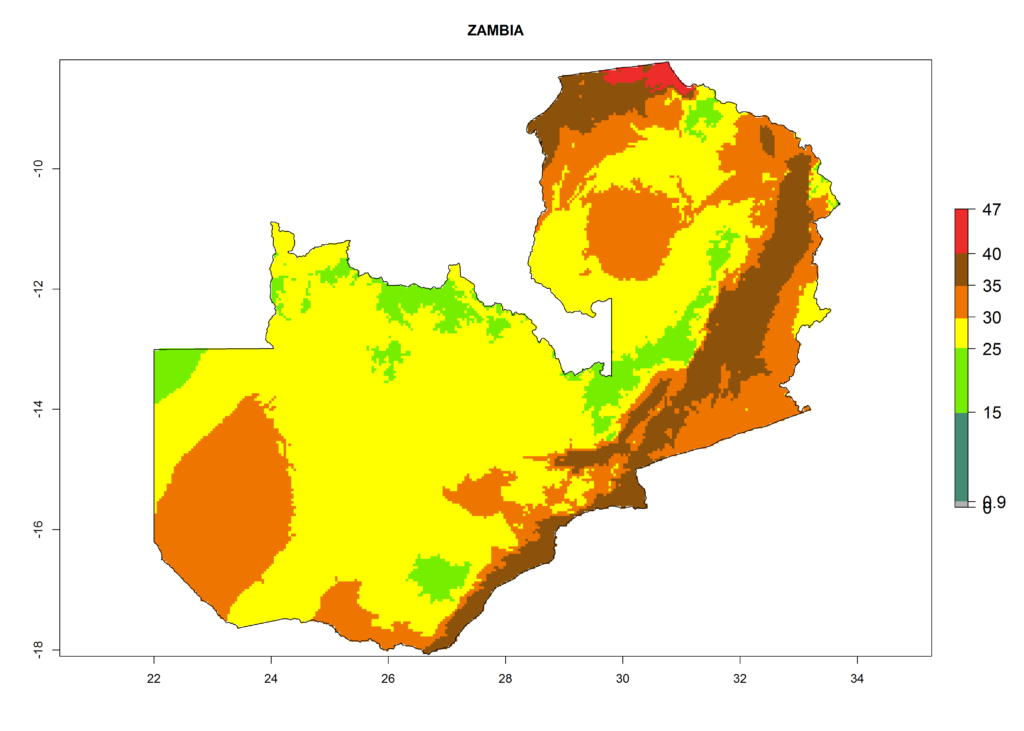
The selected national risk maps comprise African countries where L. trifolii has already been established with a very high probability of establishment (ERI>0.95–1) as in West (Benin, Ivory Coast, Guinea, Senegal, Nigeria) and East (Ethiopia, Kenya, Uganda Tanzania, Madagascar, Zimbabwe, Sudan) Africa, or with low probability (ERI>0.6–0.8) as in North (coast of Morocco, Tunisia, Egypt) and Southern (Zimbabwe and South Africa) Africa (Fig. 6). For northern and southern African countries, and highlands of East Africa, the lowest abundance (GI<20 generations per year) and potential population growth (AI<25) for L. trifolii are predicted.
| ERI | GI | AI |
a) Benin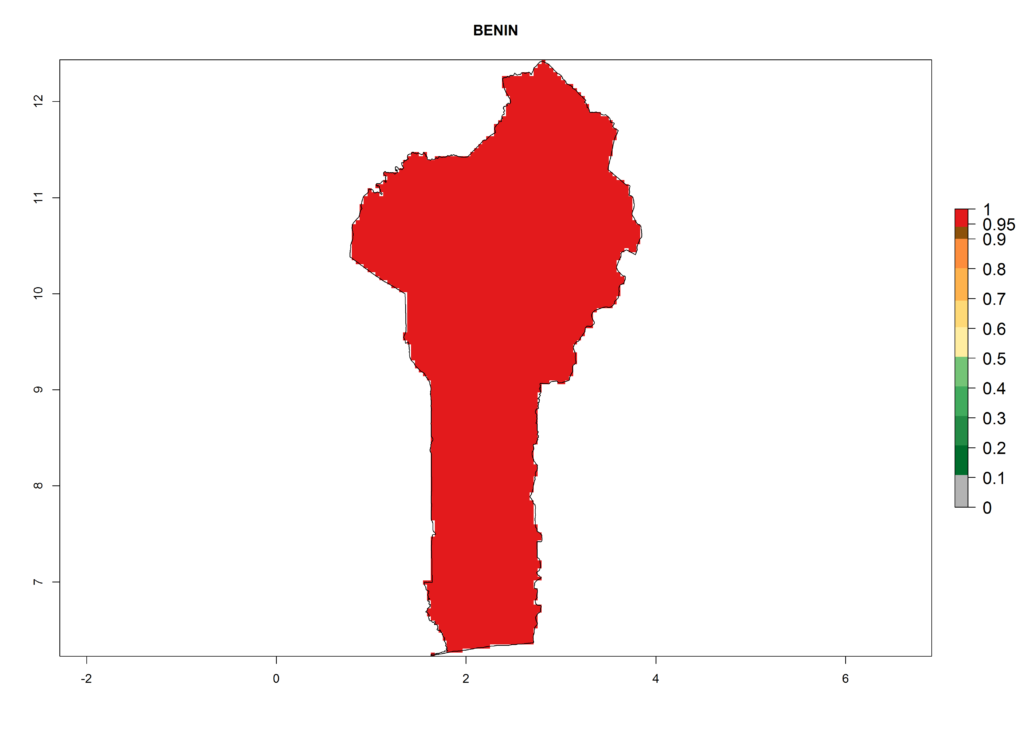 |
 |
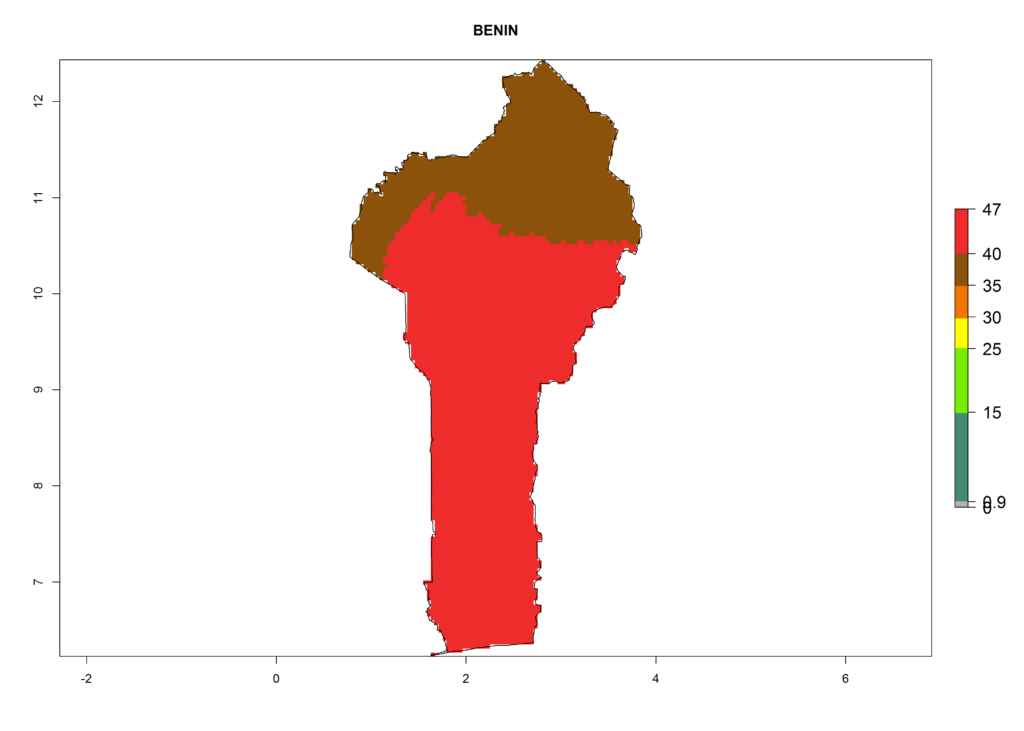 |
b) Egypt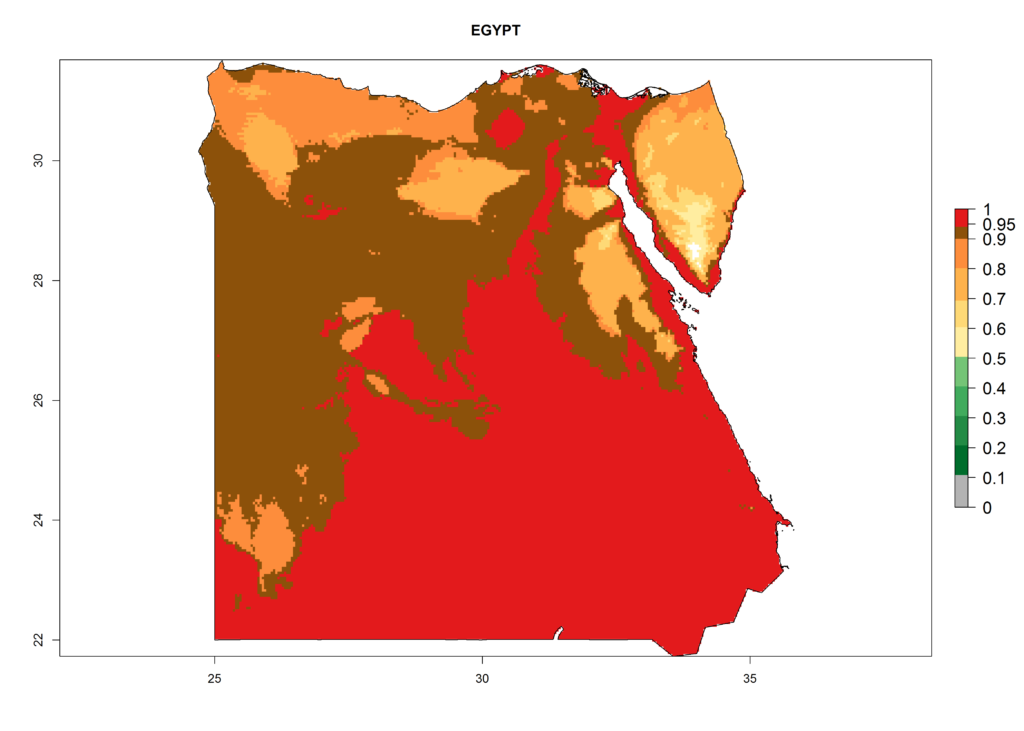 |
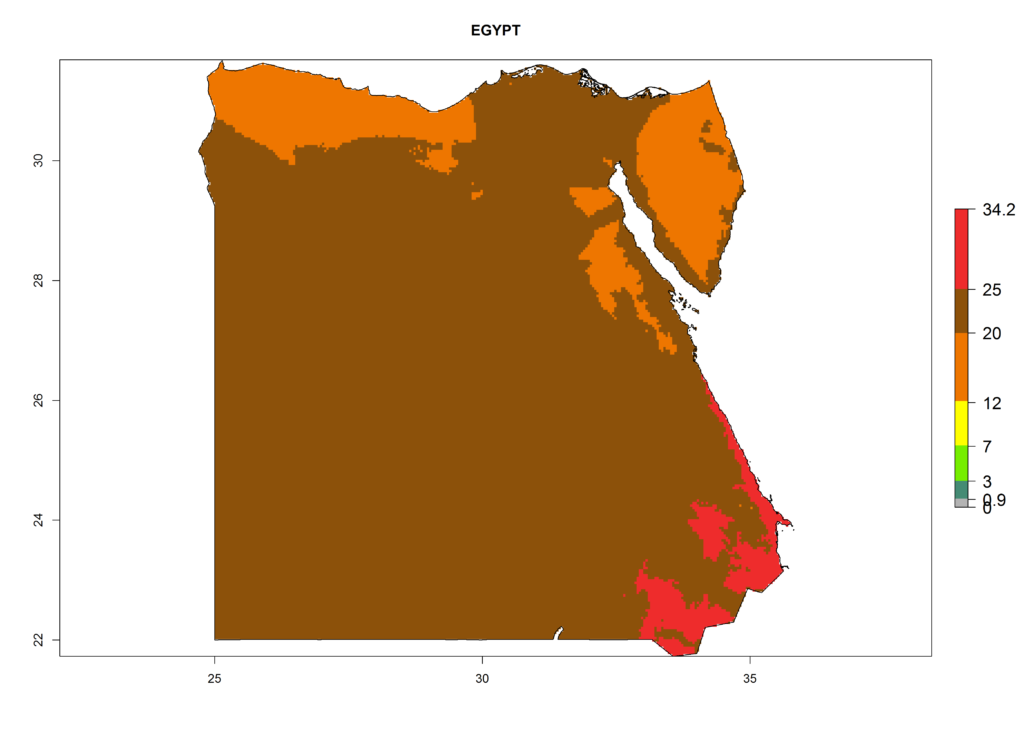 |
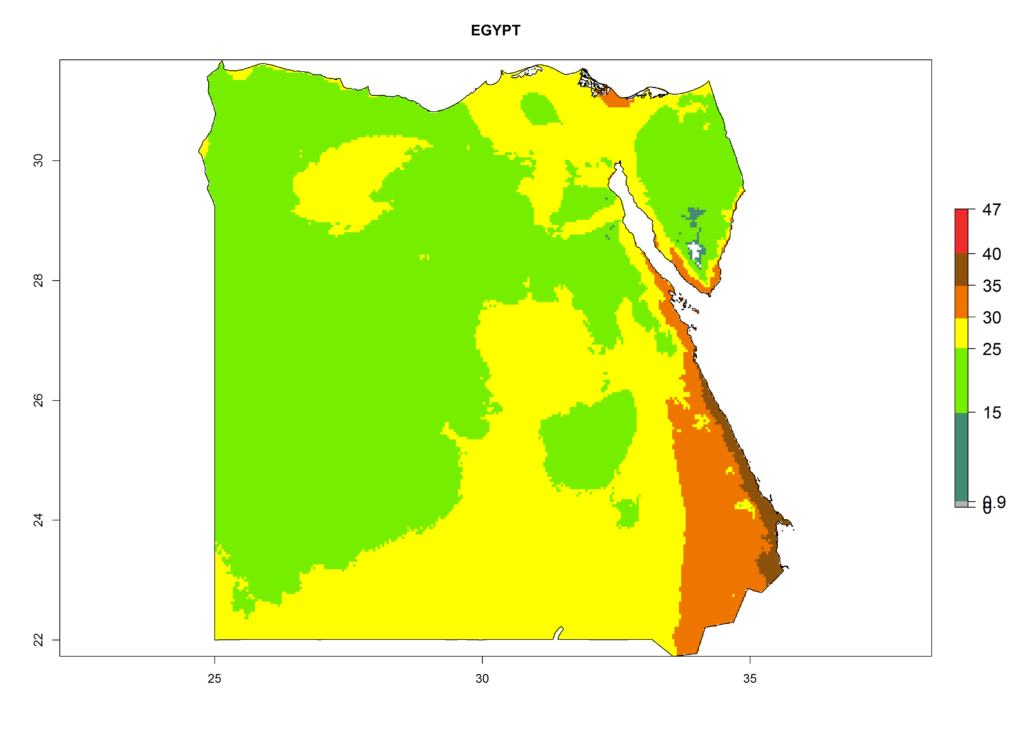 |
c) Ethiopia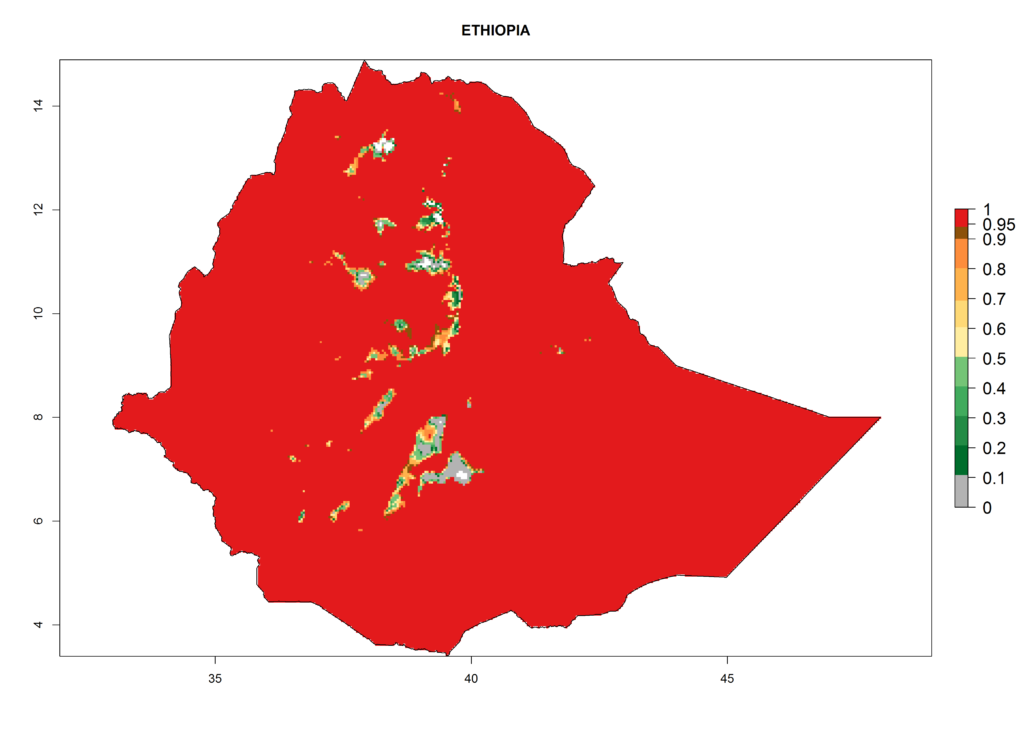 |
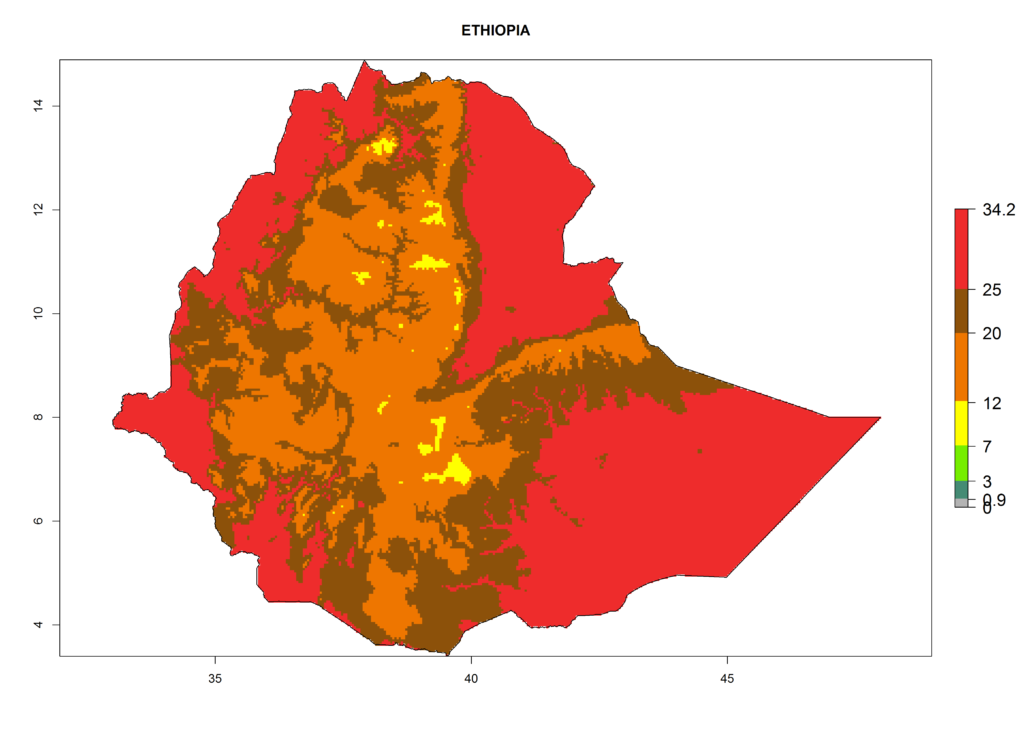 |
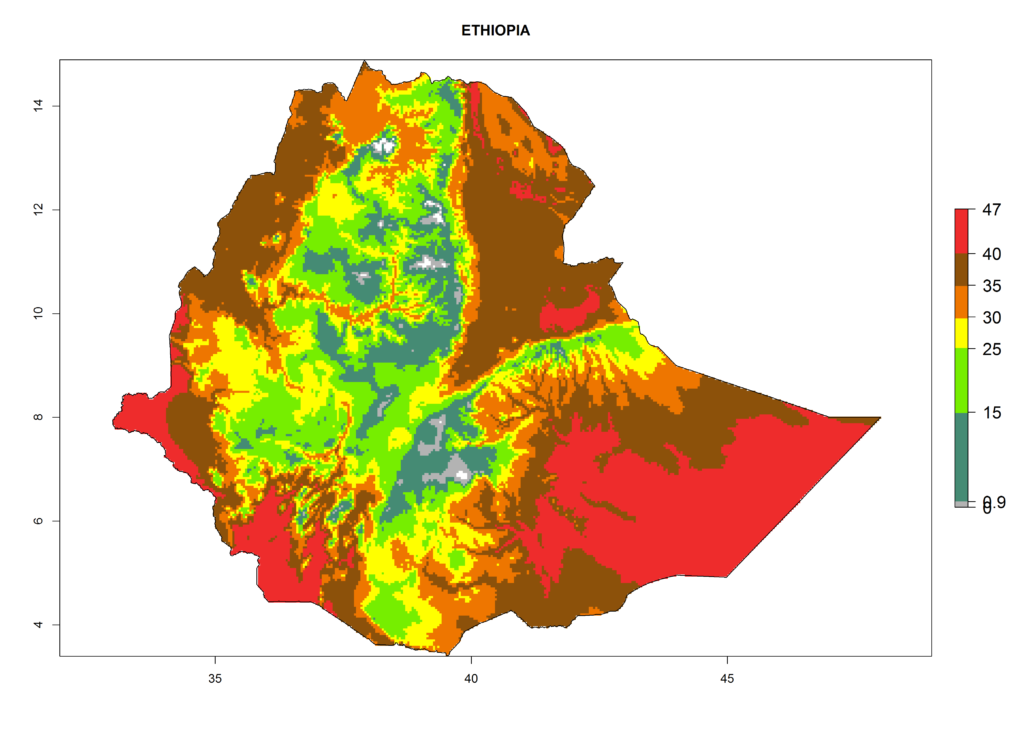 |
d) Guinea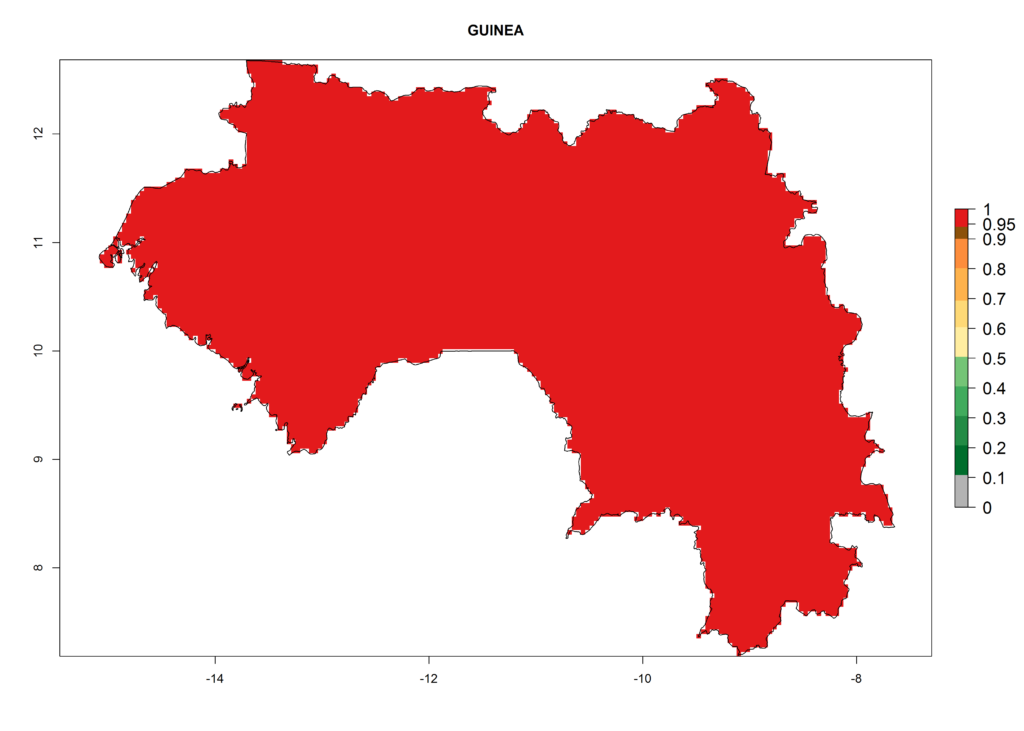 |
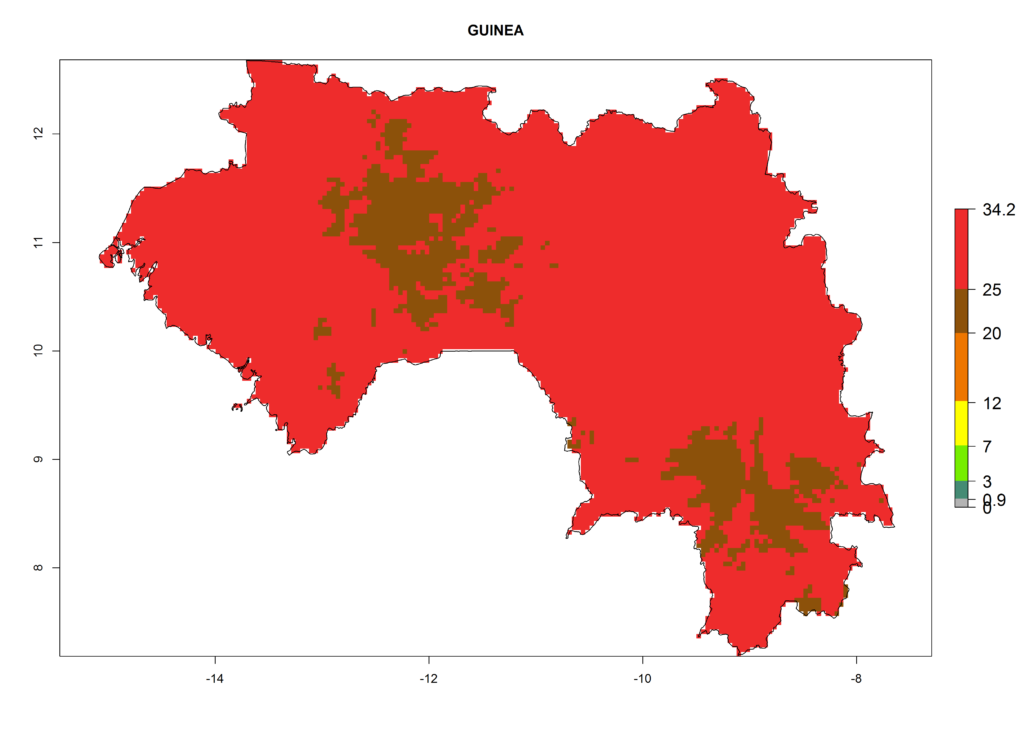 |
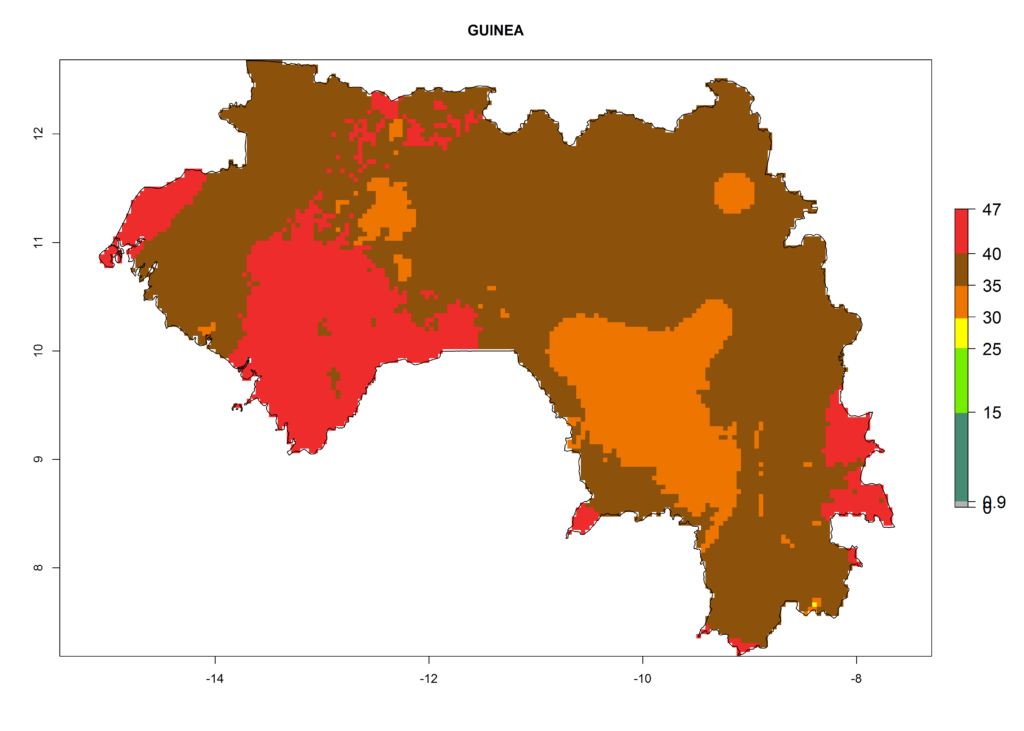 |
e) Ivory Coast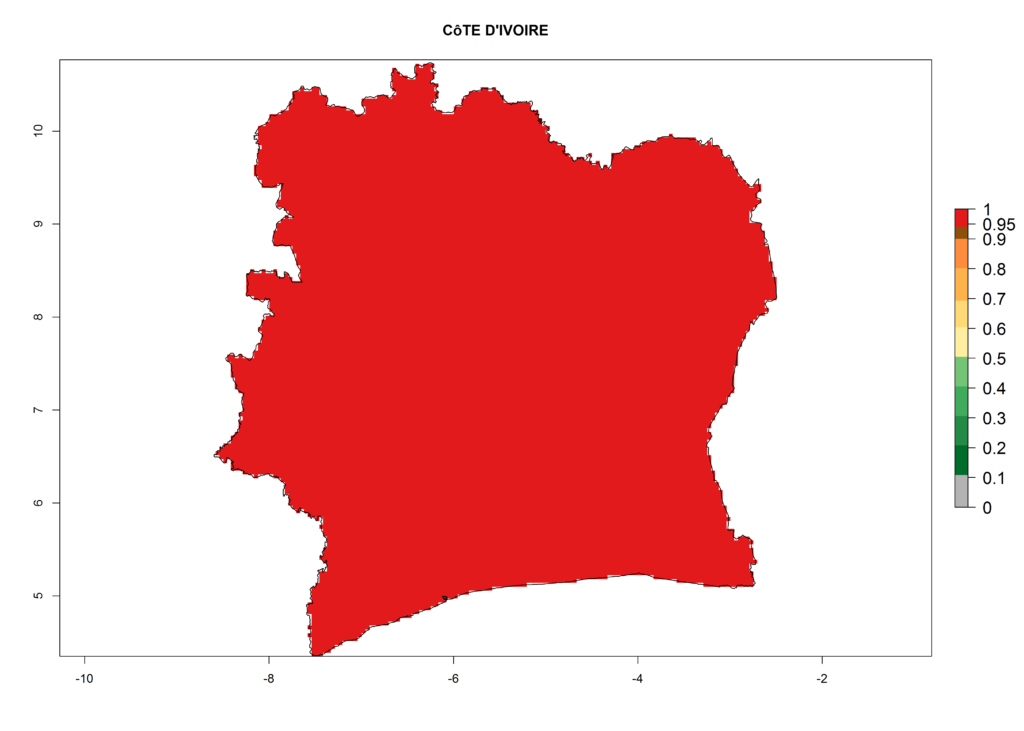 |
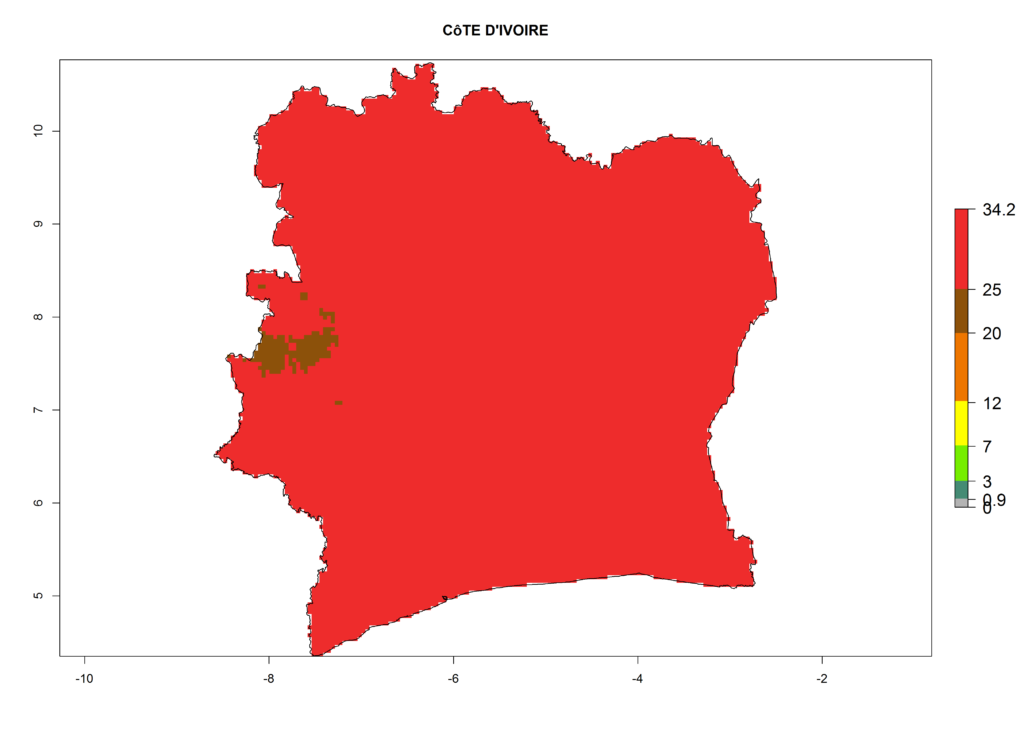 |
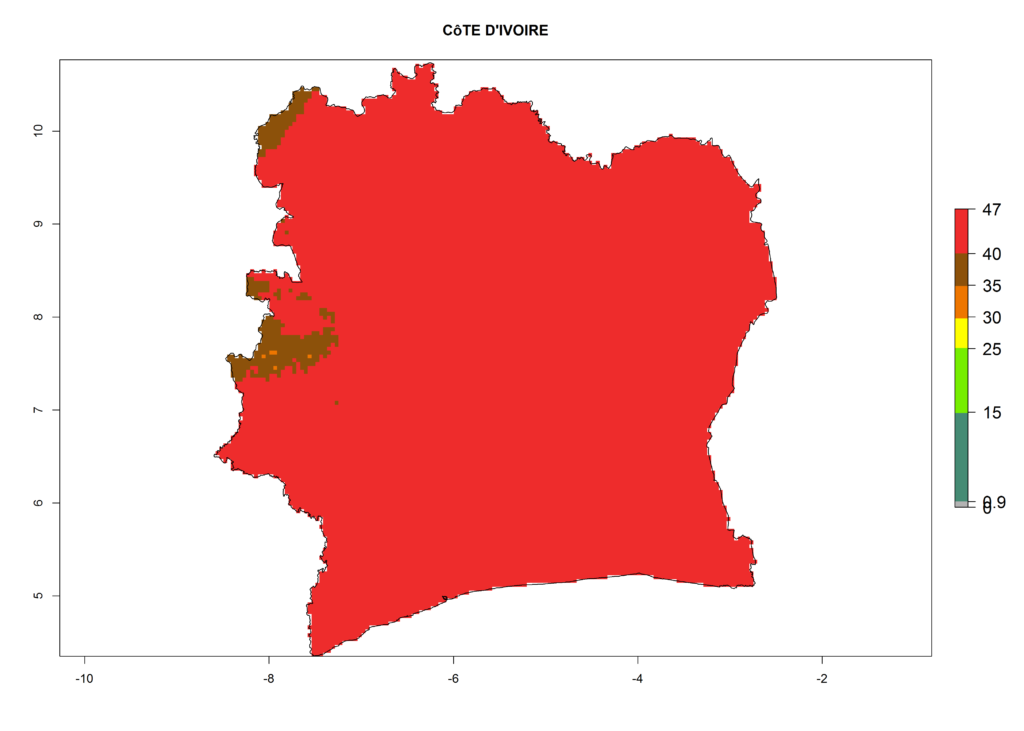 |
f) Kenya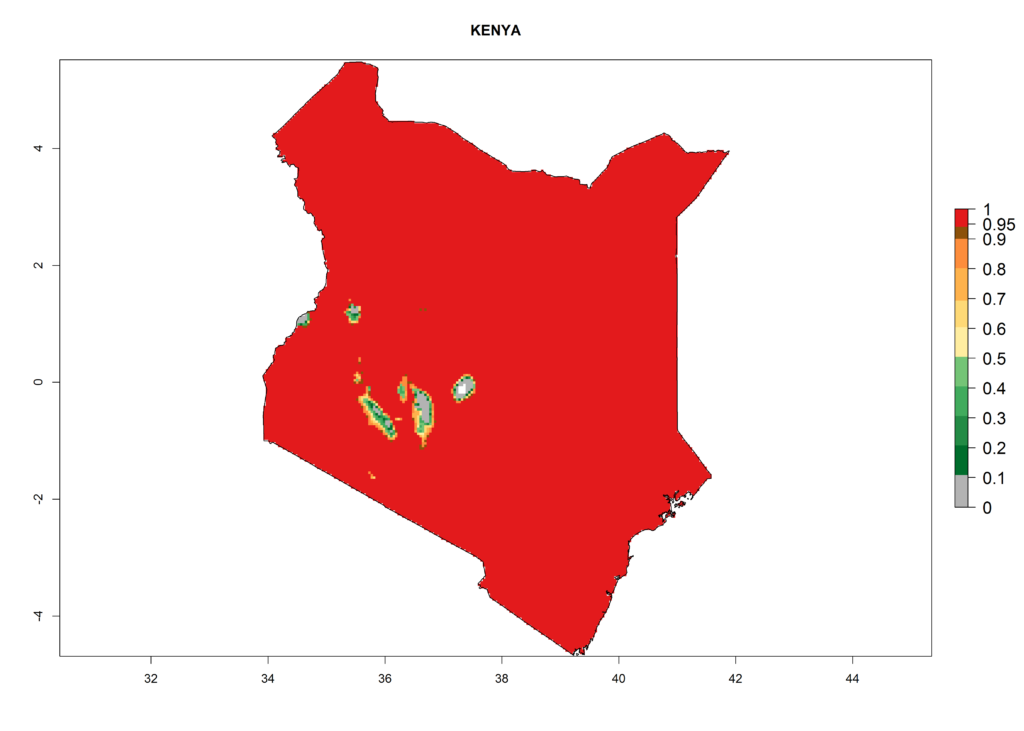 |
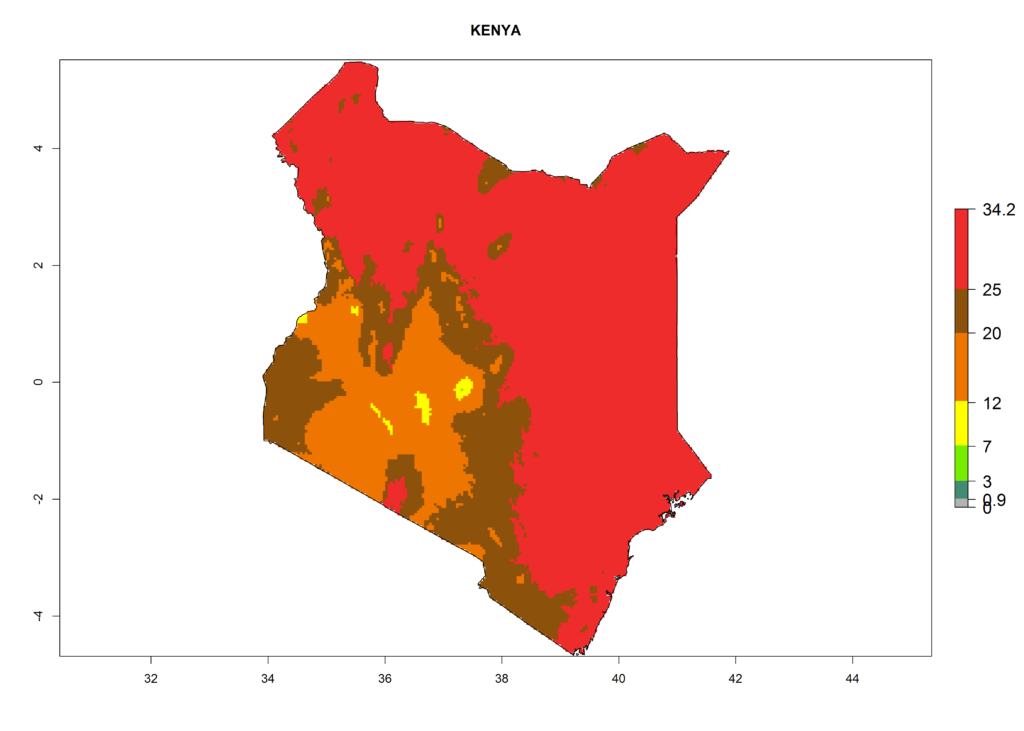 |
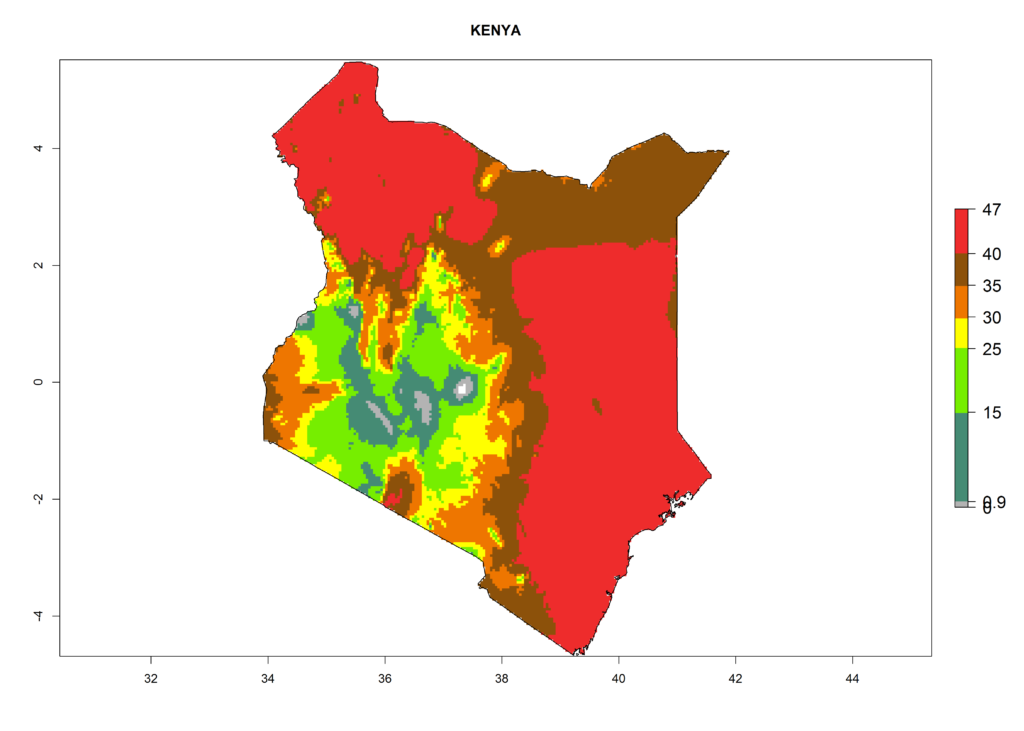 |
g) Madagascar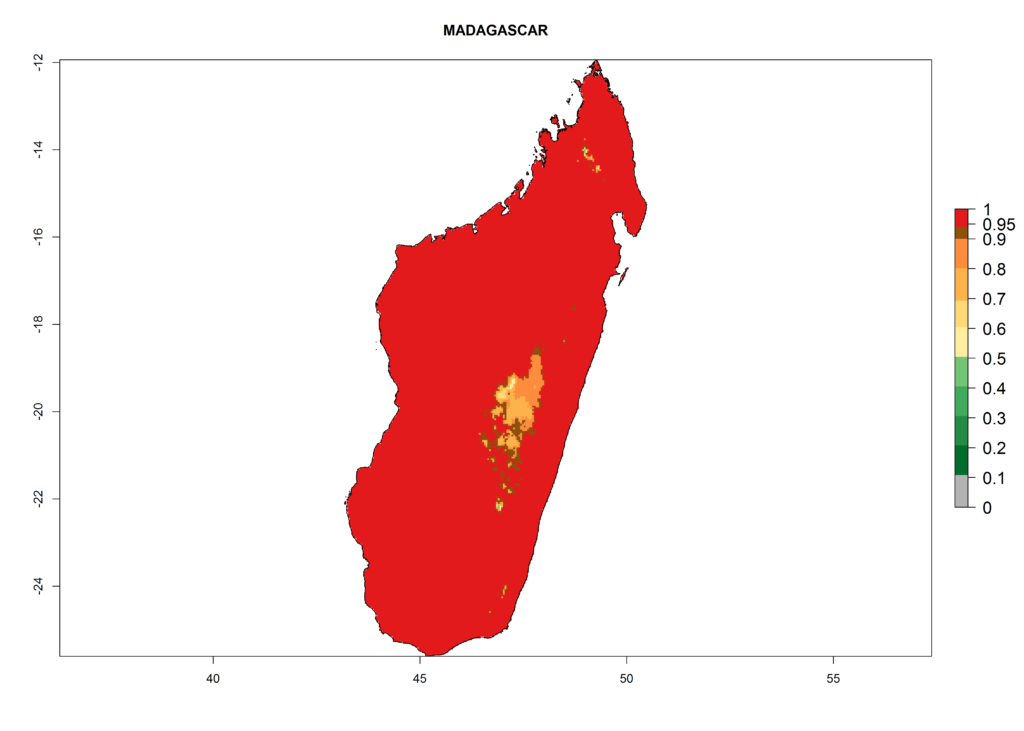 |
 |
 |
h) Morocco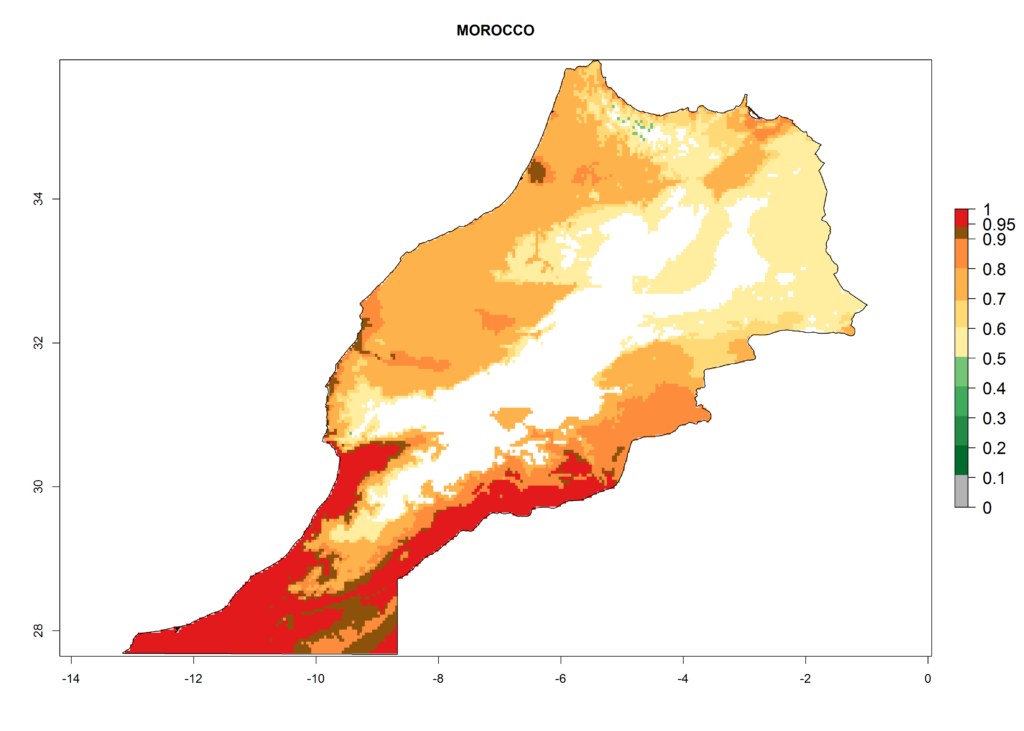 |
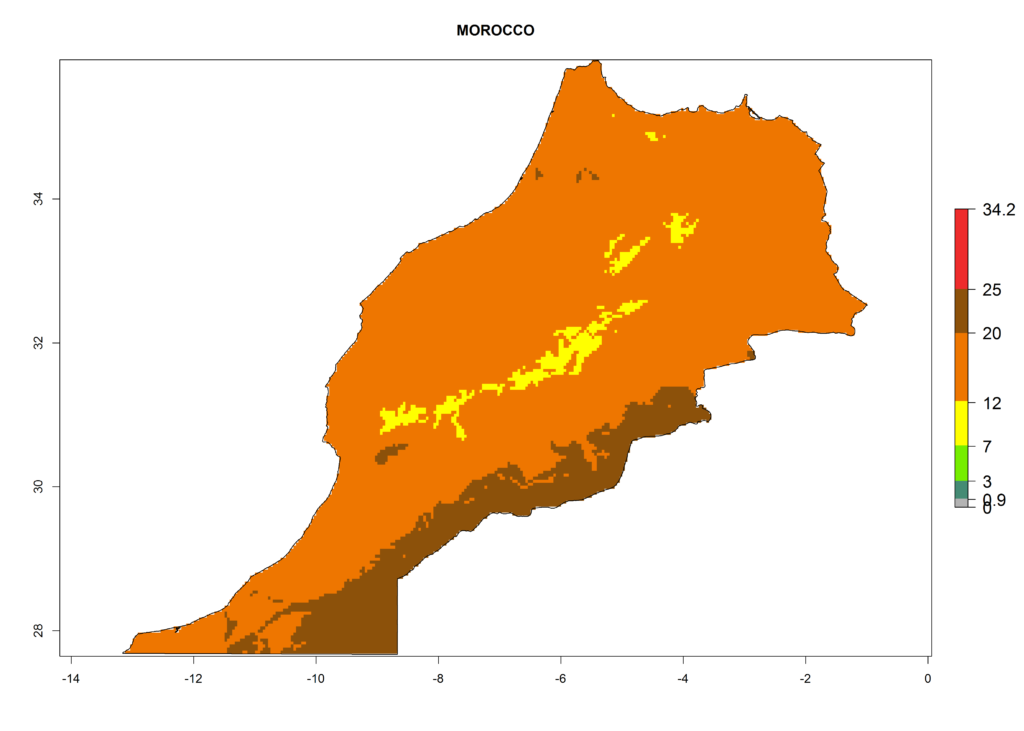 |
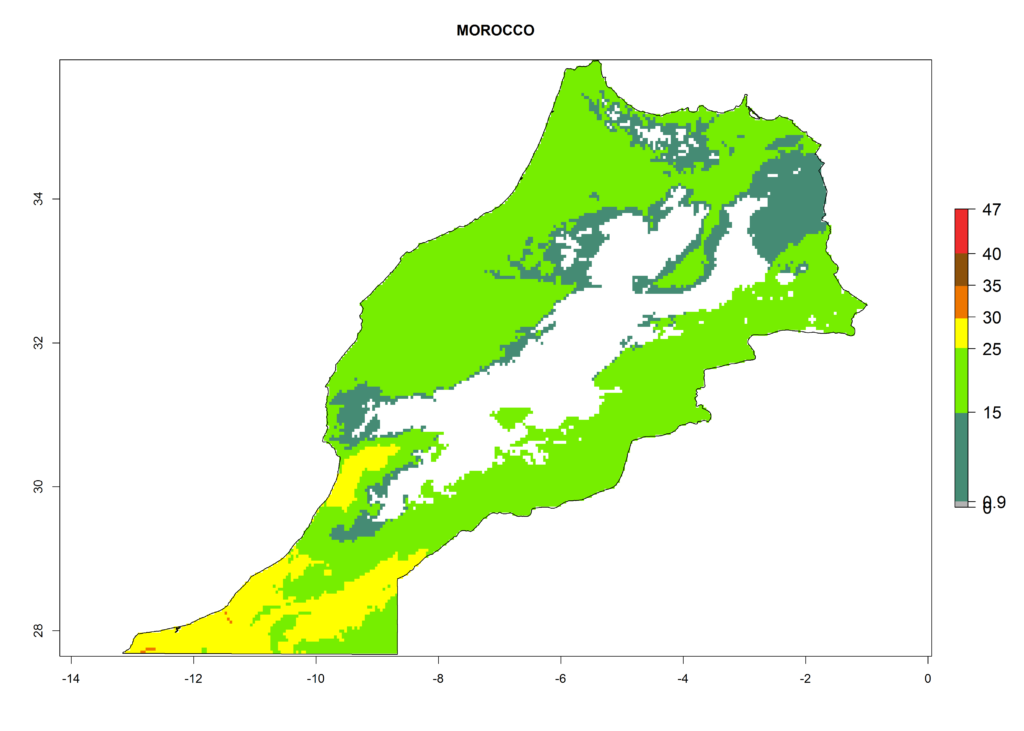 |
i) Nigeria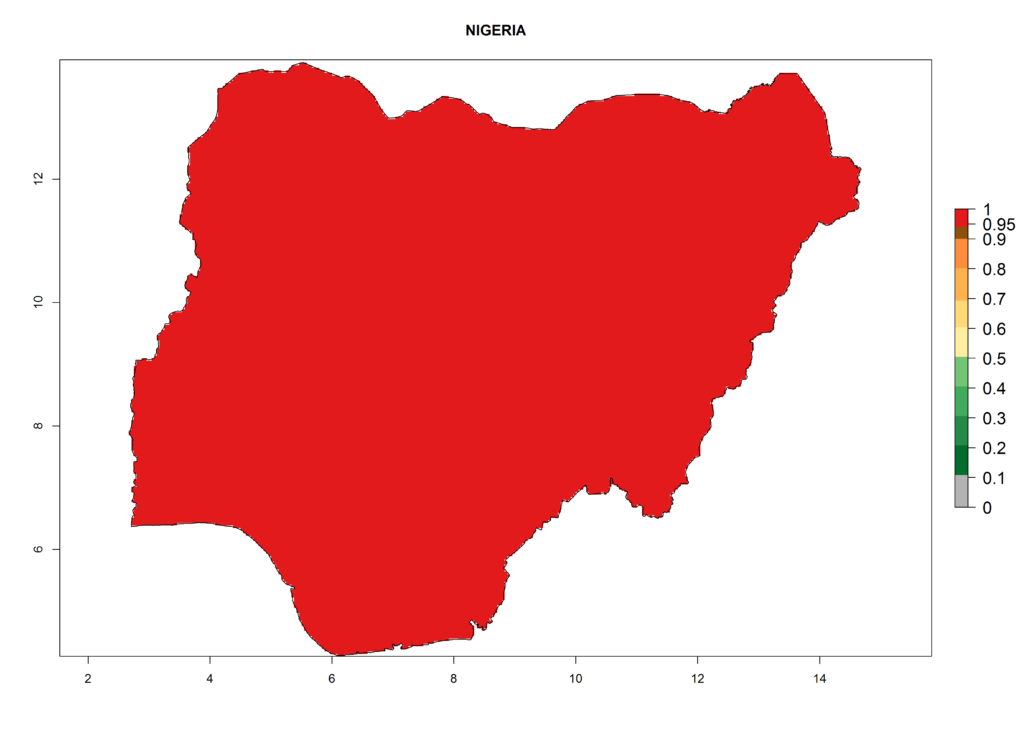 |
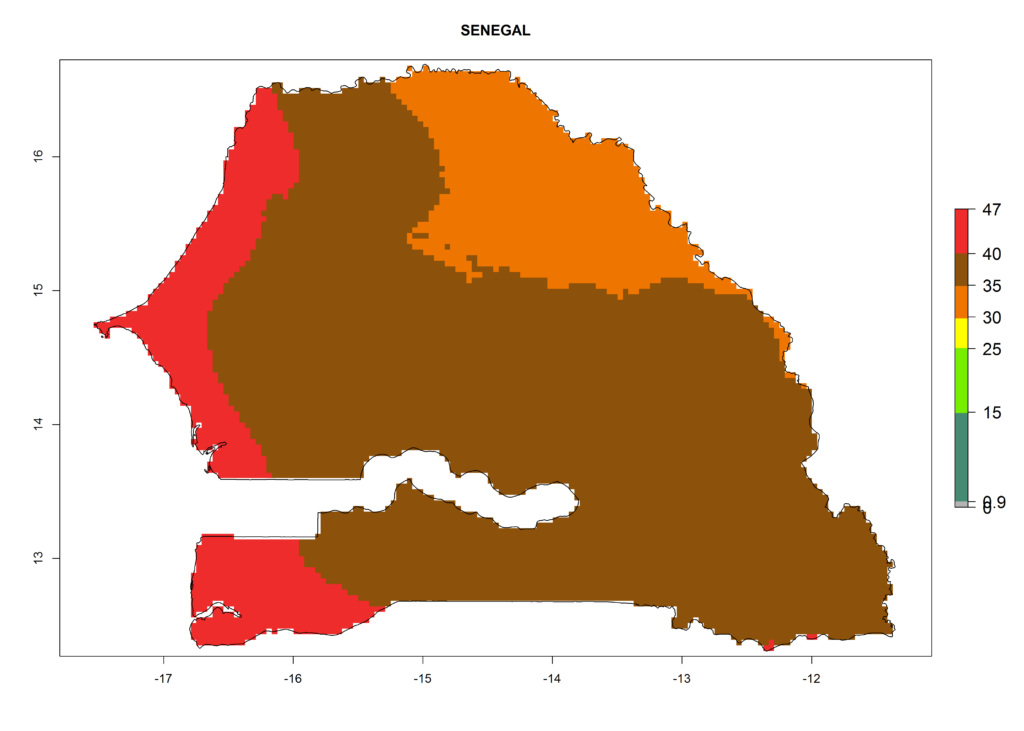
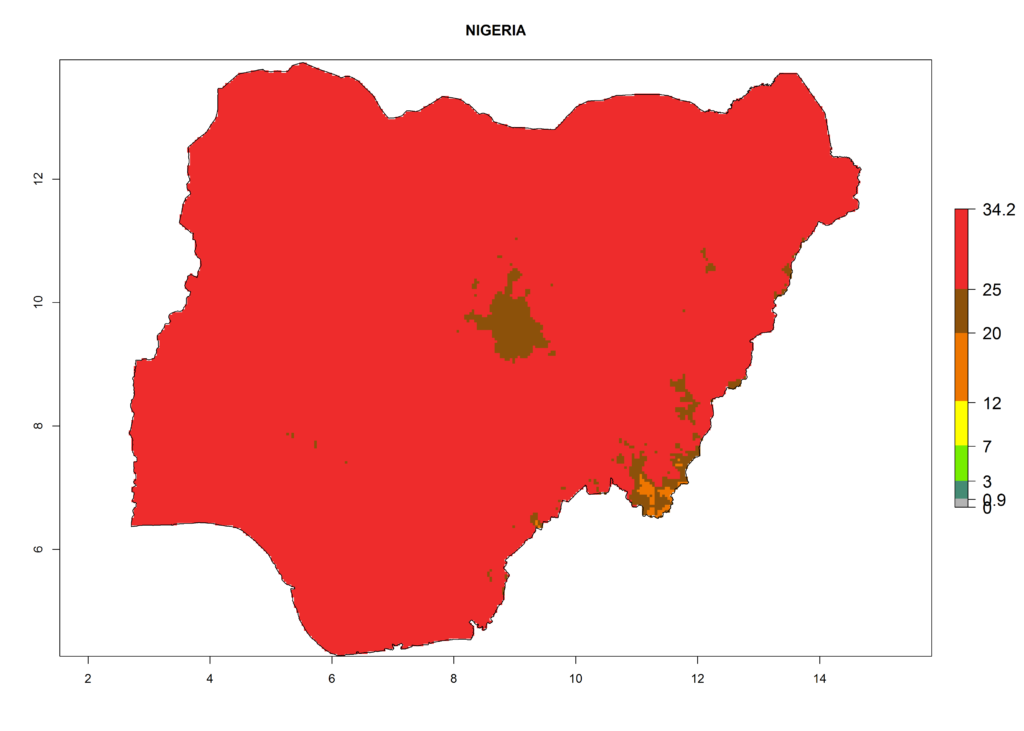 |
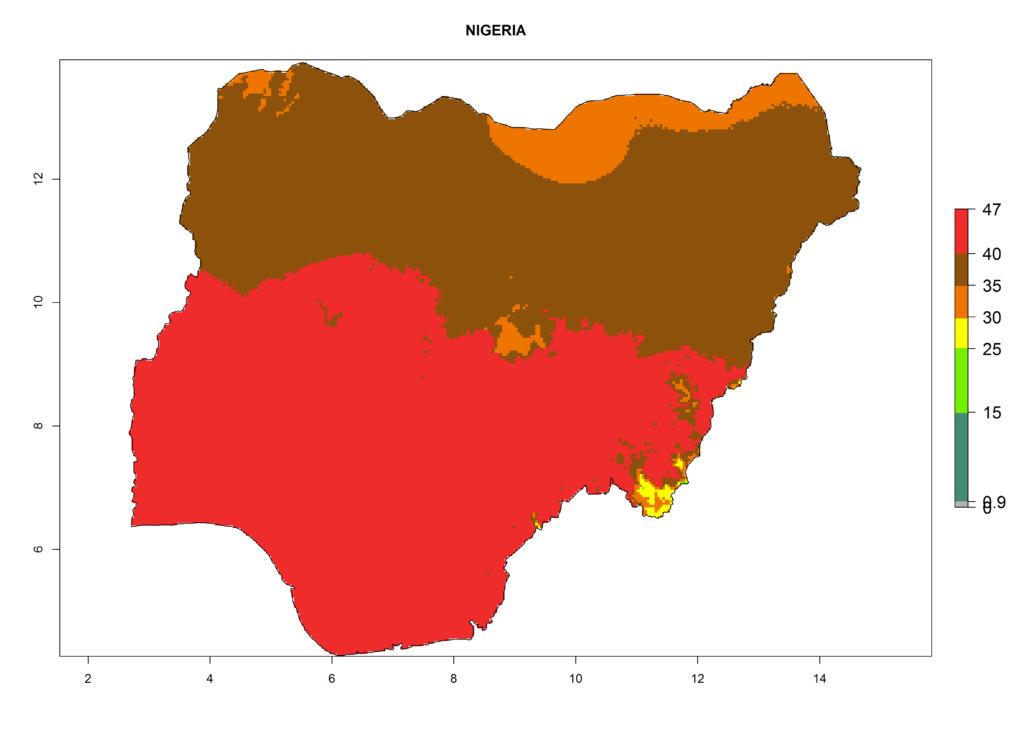 |
j) Senegal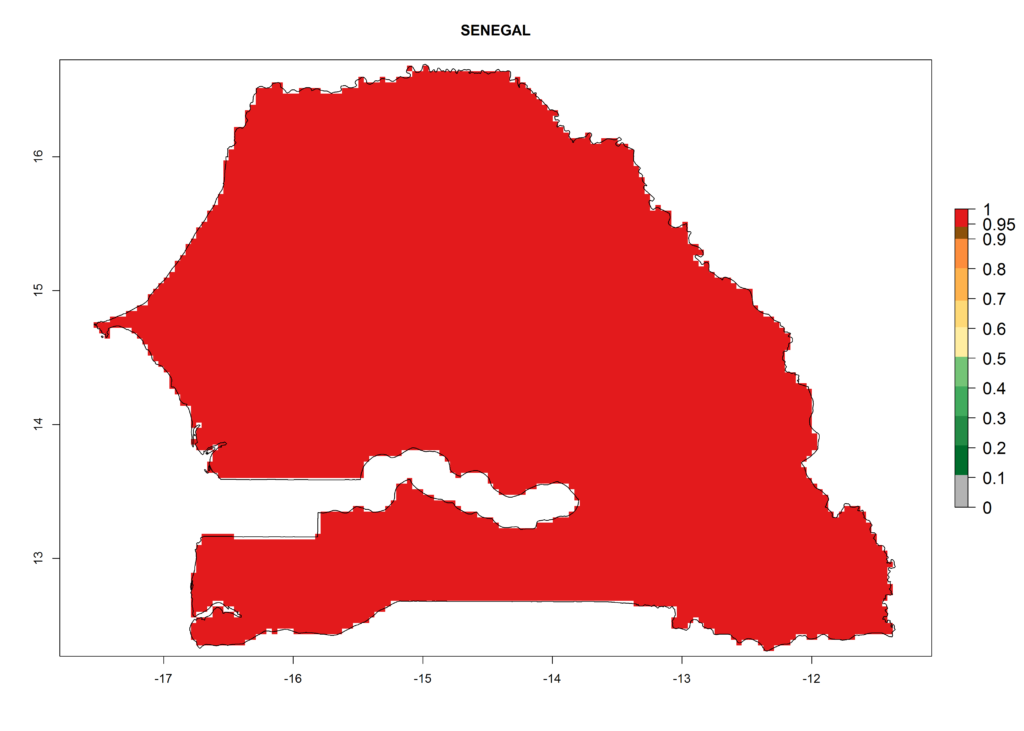 |
 |
 |
k) South Africa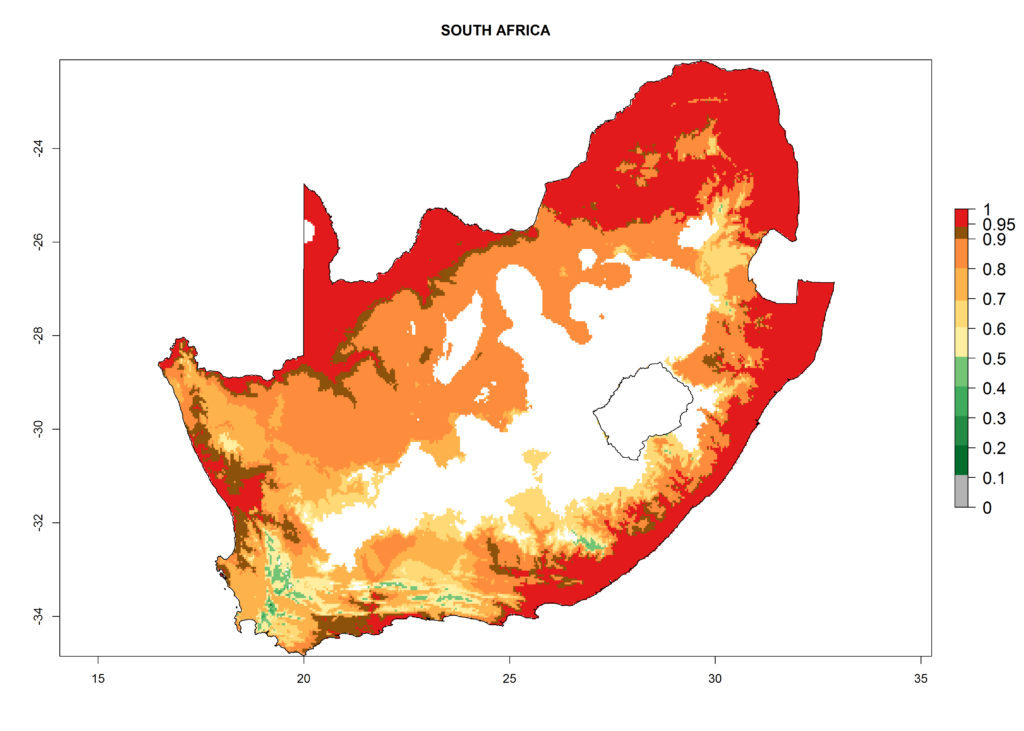 |
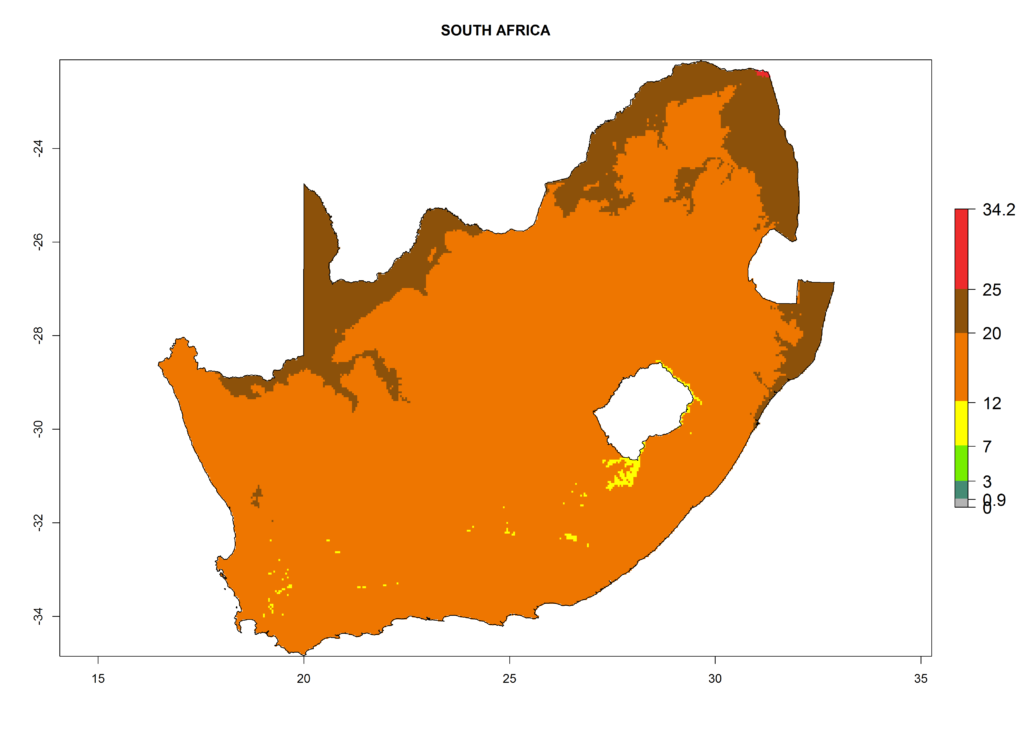 |
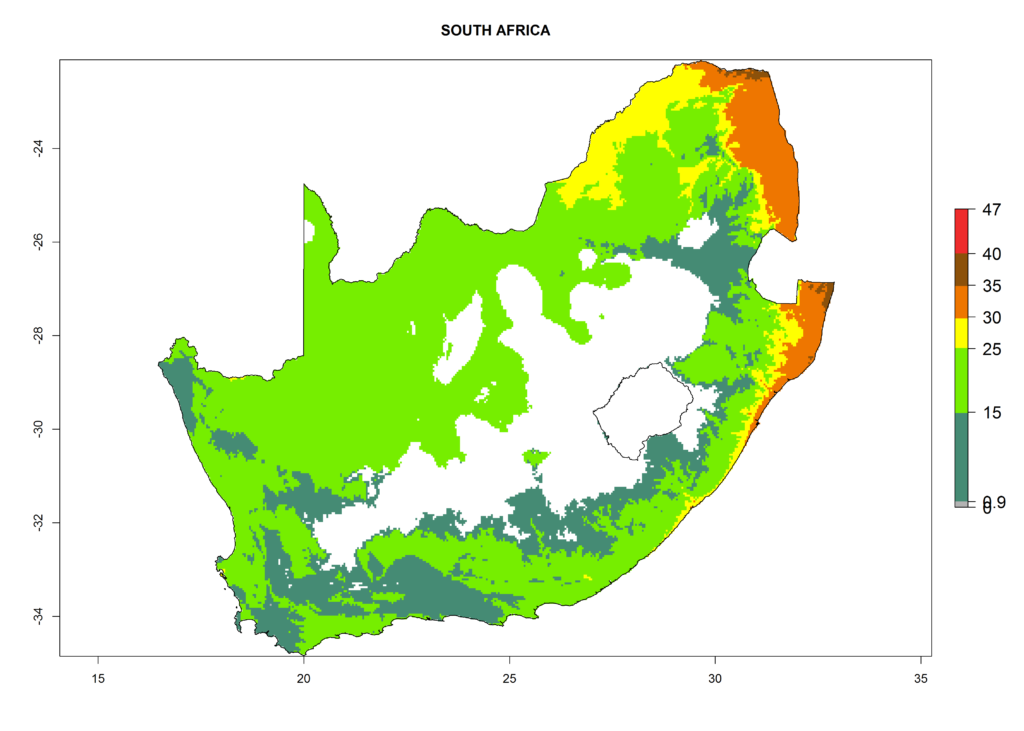 |
l) Sudan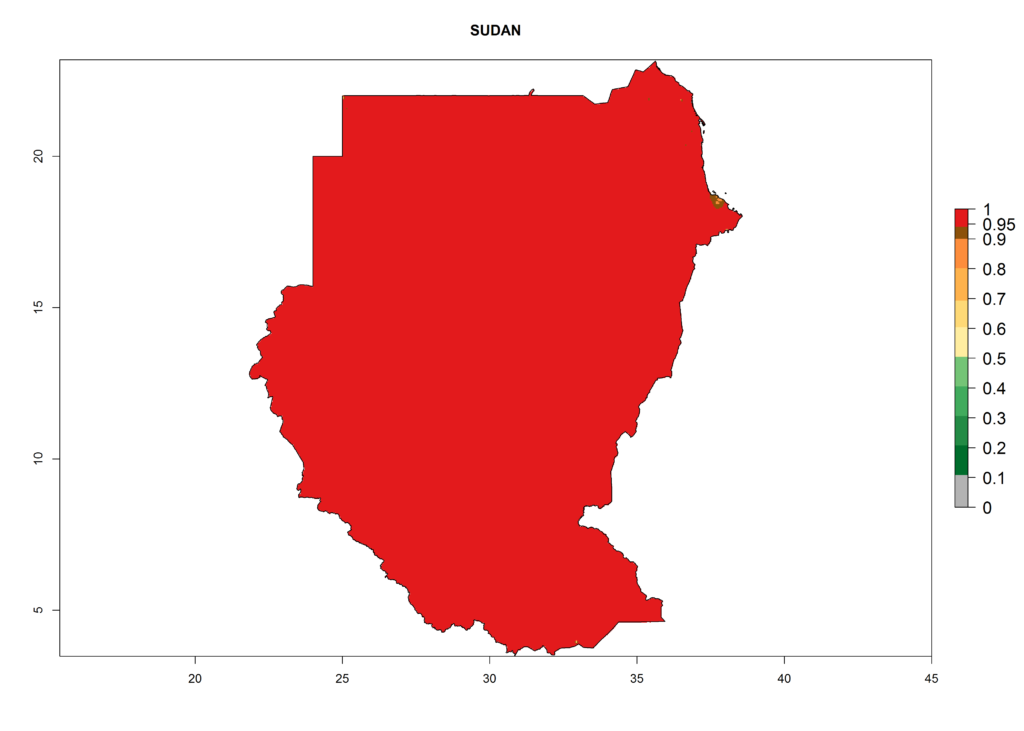 |
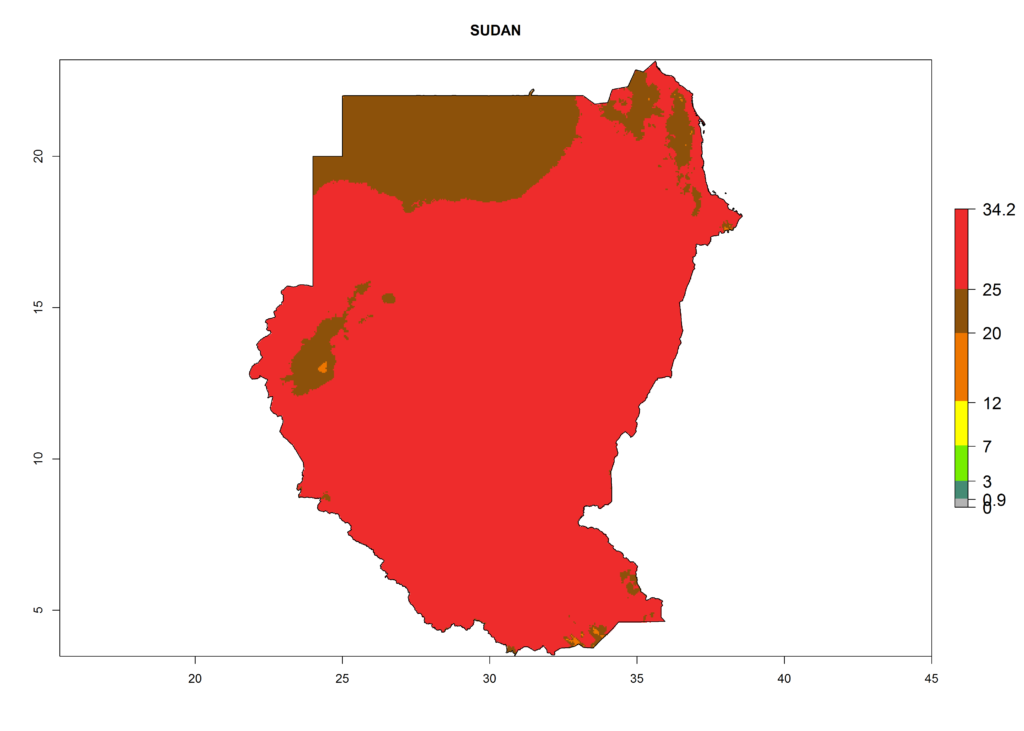 |
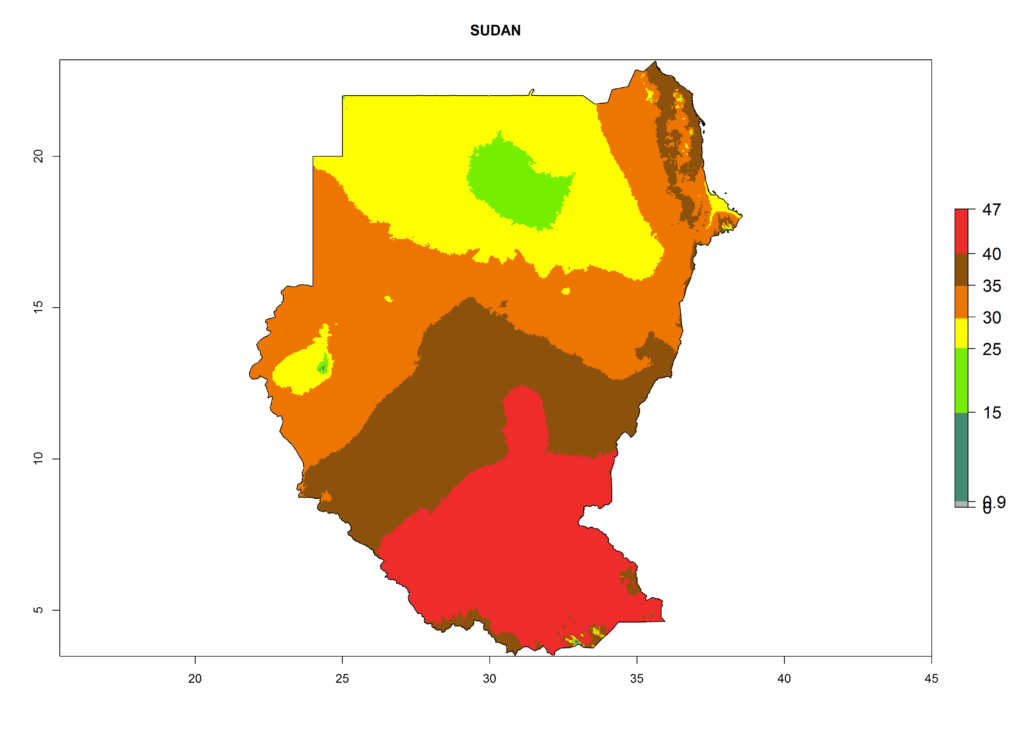 |
m) Tanzania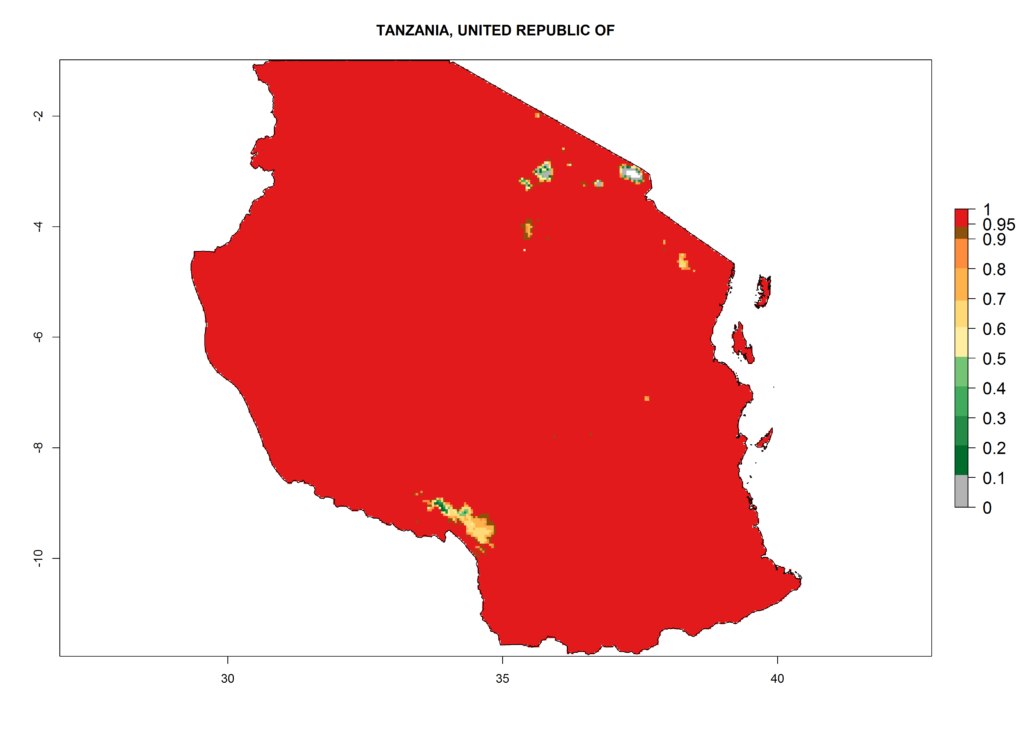 |
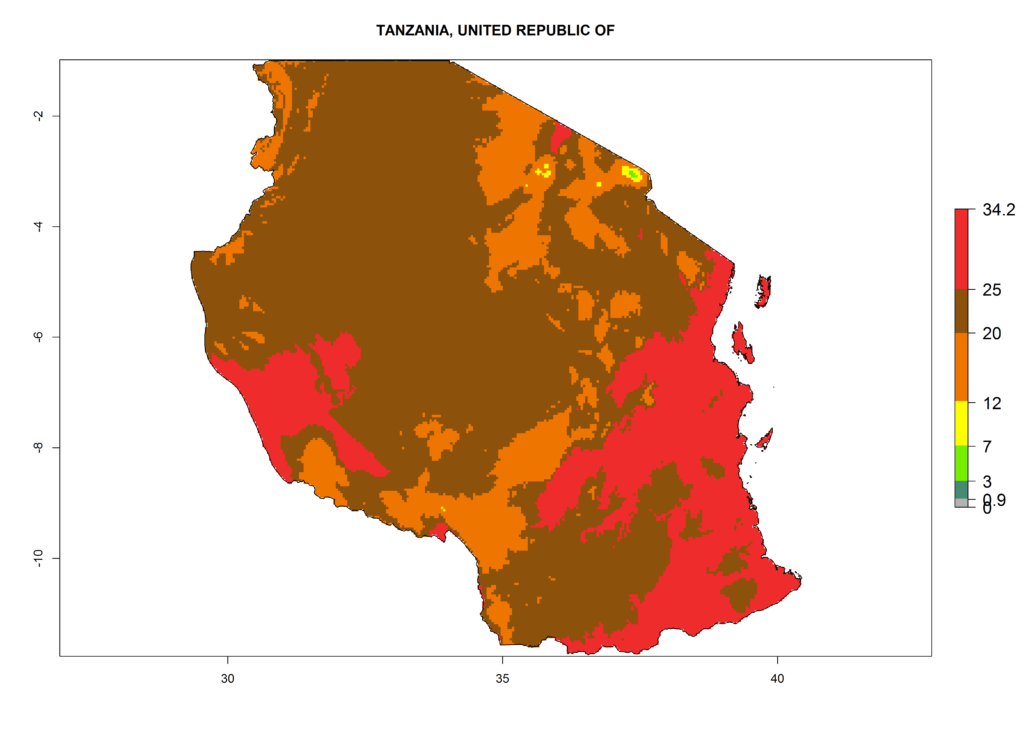 |
 |
n) Tunisia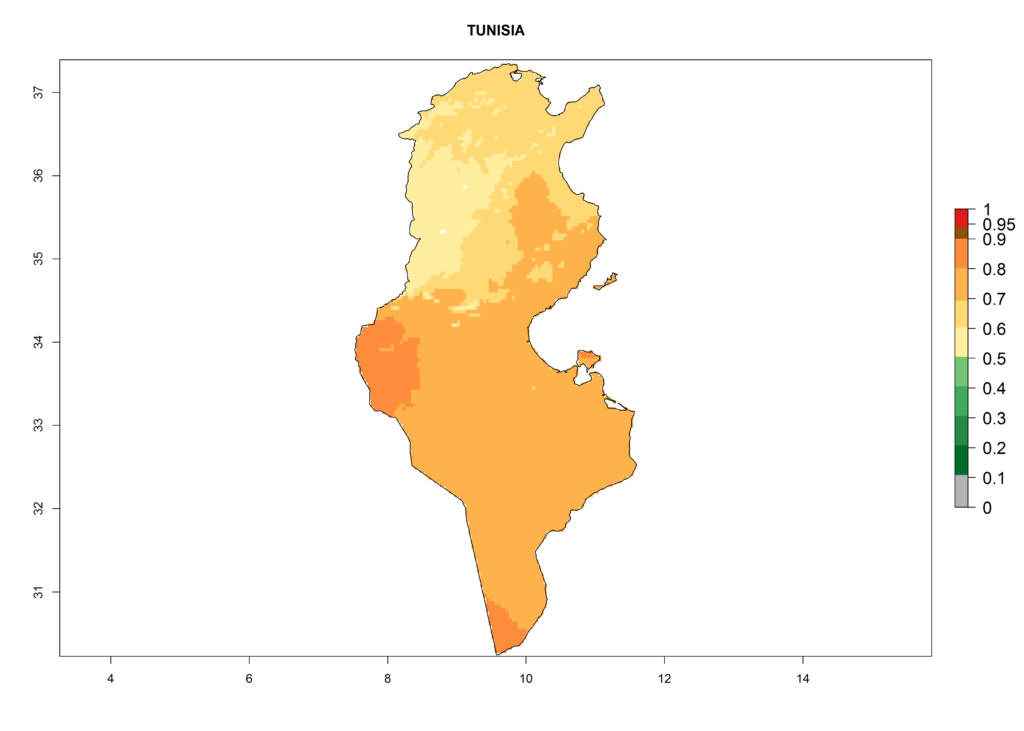 |
 |
 |
| o) Zambia | 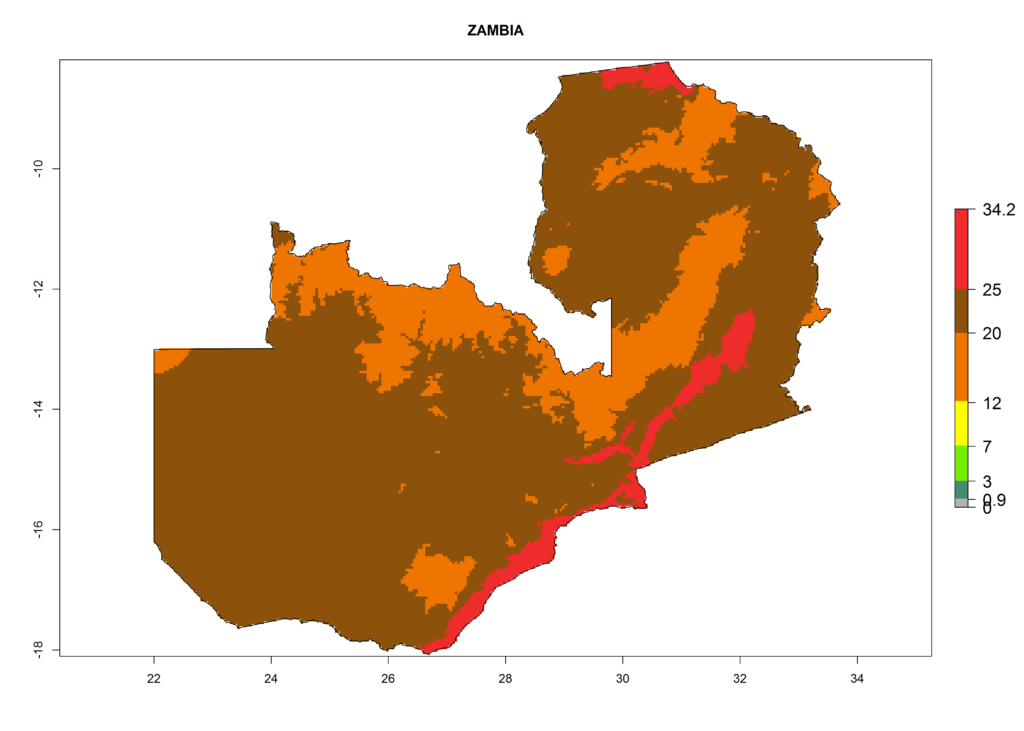 |
 |
| p) Zimbabwe | 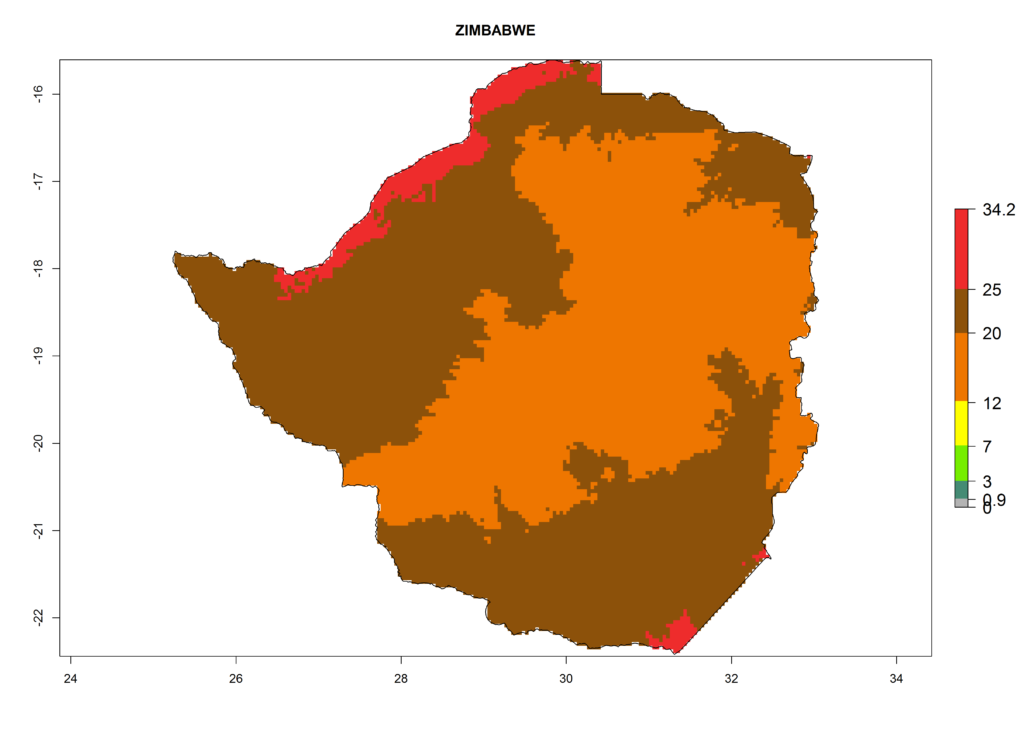 |
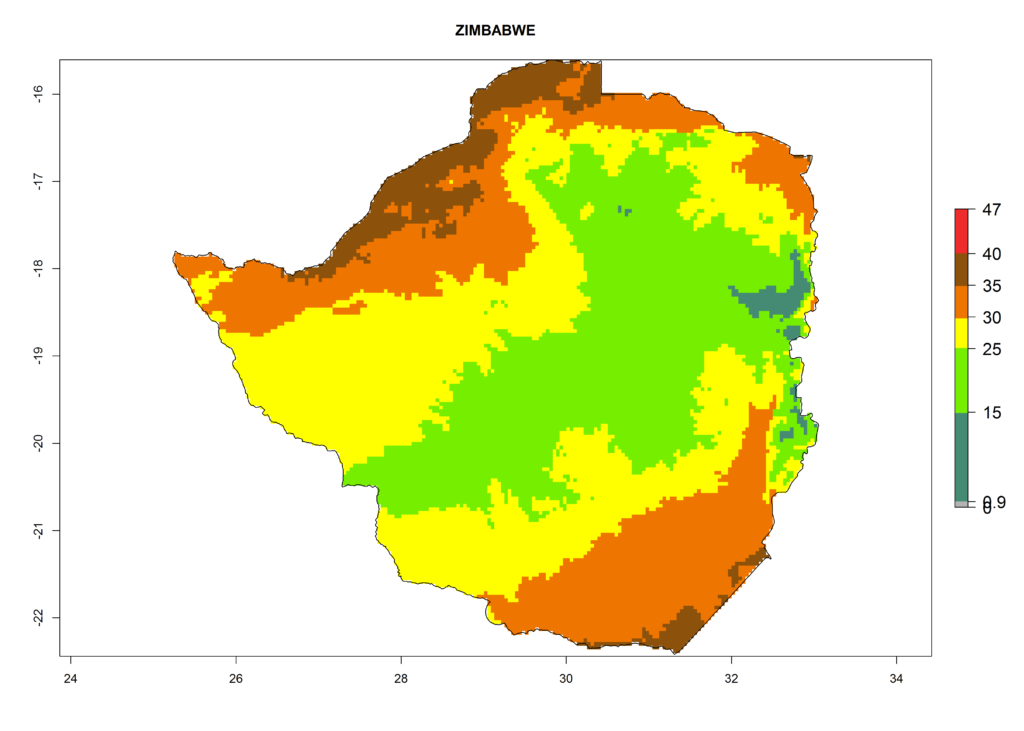 |
Figure 6. ERI, abundance (GI, potential damage), and activity (AI, potential population growth) of the American leafminer fly, Liriomyza trifolii, in selected African countries according to model predictions for the year 2000. An ERI>0.6 is associated with potential permanent establishment.
Phytosanitary measures
In chrysanthemum cuttings, L. trifolii survived cold storage at 1.7°C for at least 10 days. Newly laid eggs of L. trifolii in chrysanthemums survived for up to 3 weeks in cold storage at 0°C. Eggs without protection in plants and incubated for 36–48 hr died after 1 week under the same conditions. All larvae instars died after 1–2 weeks at 0°C. Hence, it is recommended to place chrysanthemum cuttings under normal glasshouse conditions for 3–4 days after lifting to allow eggs to hatch with a subsequent storage period of 1–2 weeks at 0°C. Gamma irradiation of eggs and first larval stages at doses of 40–50 Gy provided effective control, but lower doses were ineffective. The EPPO recommends that planting material (except seeds) of celery, Cucumis, lettuce and tomato, and material (except seeds and pot plants) of Capsicum, carnation, chrysanthemum, Gerbera, Gypsophila, and Senecio hybridus from countries where the pest occurs must either have been inspected at least every month during the previous 3 months and found free from the pests, or have been treated by a recommended method. Whether countries make the same requirements for potted plants of this second group of plants is optional. A phytosanitary certificate may be required for cut flowers and for vegetables with leaves.
Adaptation to risk avoidance at farm level
Management strategies are similar for all different species of Liriomyza attacking vegetables, which are described in section 4.3.1, L. huidobrensis.
Sampling. Methods of sampling include counting live larvae and/or mines, collecting pupae, and rearing adults from foliage to monitor larvae infestation and infestation dynamics. Counting the number of flies captured in yellow sticky traps monitors adult leafminer fly activity. Both methods can be adapted for decision-making to avoid unnecessary applications of insecticides.
Cultural/mechanical control. Sanitation, including the removal of infested plants after harvests and weeds, is important because L. trifolii is polyphagous. Sanitation will prevent the build-up of pest populations in these reservoirs. There has been limited success in using trap plants in covered crops.
Plant resistance. Research on breeding for resistance focused on developing plants with a high density of glandular trichomes, which will physically reduce feeding and restrict oviposition sites in tomatoes. Other crops, such as lettuce, spinach, and celery, are being evaluated for resistance.
Chemical control. Although the pest’s adults are susceptible to many insecticides, it is preferred to apply translaminar insecticides (abamectin, cyromazine, neem, or spinosad) to control larvae. Applications should be carefully timed, because the pest is most susceptible at an early larval stage. There are indications of incipient resistance to these pesticides reported.
Biological control. About 30 hymenopterous parasitoids attack L. trifolii worldwide. In the Middle East the more important species are Diglyphus isaea Walker, D. crassinervis Erdos, and Neochrysocharis formosa (Westwood) (all Eulophidae), which control the pest on various crops. Some success has been achieved in Europe by applying entomopathogenic nematodes, such as Steinernema feltiae, for leafminer control.
Further reading
CABI. 2014c. Liriomyza trifolii, American serpentine leafminer. Invasive Species Compendium: Datasheets, maps, images, abstracts and full text on invasive species of the world. Available from http://www.cabi.org/isc/datasheet/30965
Dempewolf, M. 2004. Arthropods of Economic Importance – Agromyzidae of the World (CD-ROM). ETI. University of Amsterdam, Netherlands. http://wbd.etibioinformatics.nl/bis/ agromyzidae.php
European Food Safety Authority (EFSA). 2012. Panel on Plant Health (PLH); Scientific Opinion on the risks to plant health posed by Liriomyza huidobrensis (Blanchard) and Liriomyza trifolii (Burgess) to the EU territory with the identification and evaluation of risk reduction options. Available from: www.efsa.europa.eu/efsajournal
European Plant Protection Organisation (EPPO). 2014a. Data Sheets on Quarantine Pests: Liriomyza trifolii, 6 pp. Prepared by CABI and EPPO for the EU under Contract 90/399003. Available from http://www.eppo.int/QUARANTINE/insects/Liriomyza_trifolii/LIRITR_ds.pdf
Foster, R.E., and C.A. Sanchez. 1988. Effect of Liriomyza trifolii (Diptera: Agromyzidae) larval damage on growth, yield and cosmetic quality of celery in Florida. Journal of Economic Entomology 81: 1721–1725.
Gencsoylu, I. 2003. Note: A new pest, Liriomyza trifolii, on cotton in the Buyuk Menderes Valley, Turkey. Phytoparasitica 31: 330–332.
Harris, H.M., J.W. Begley, and D.L. Warkentin. 1990. Liriomyza trifolii (Diptera: Agromyzidae) suppression with foliar applications of Steinernema carpocapsae (Rhabditida: Steinernematidae) and abamectin. Journal of Economic Entomology 83: 2380–2384.
http://www.eppo.int/QUARANTINE/insects/Liriomyza_trifolii/LIRITR_ds.pdf
Lanzoni, A., G.G. Bazzocchi, G. Burgi, and M.R. Fiacconi. 2002. Comparative life history of Liriomyza trifolii and Liriomyza huidobrensis (Diptera: Agromyzidae) on beans: Effect of temperature on development. Environmental Entomology 31: 797–803.
Leibee, G.L. 1982. Development of Liriomyza trifolii on celery. In: Proceedings of IFAS-Industry Conference on Biology and Control of Liriomyza leafminers. D.J. Schuster, ed., 35–41. Lake Buena Vista, Florida.
Minkenberg, O.P.J.M. 1988a. Dispersal of Liriomyza trifolii. OEPP/EPPO Bulletin 18: 173–182.
Minkenberg, O.P.J.M. 1988b. Life history of the agromyzid fly Liriomyza trifolii on tomato at different temperatures. Entomologica Experimentalis et Appliciata 48: 73–84.
Parrella, M.P., W.W. Allen, and P. Marishita. 1981. Leafminer species causes California chrysanthemum growers new problems. California Agriculture 35: 28–30.
Spencer, K.A. 1973. Agromyzidae (Diptera) of economic importance (Series Entomologica, Vol. 9), 418 pp. Dordrecht: Springer Science + Business Media.