5.1.6 Biocontrol Agents Associated With Potato And Vegetable Pests![]() / Chrysocharis flacilla (Walker 1842)
/ Chrysocharis flacilla (Walker 1842)
Synonyms: Chrysocharis (Chrysocharis) flacilla (Walker 1842)
Chrysocharis flacilla (Walker 1842)
Chrysocharis (Chrysocharis) phytomyzae (Brèthes 1923)
Chrysocharis phytomyzae (Brèthes 1923)
Entedon flacilla (Walker 1842)
Euparacrias phytomyzae (Brèthes 1923)
Paracrias phytomyzae (Brèthes 1923)
Taxonomic position: Hymenoptera, Eulophidae (Entedoninae)
Authors: N. Mujica, L. Ramirez, P. Carhuapoma, & J. Kroschel
Hosts
Chrysocharis flacilla is found parasitizing leafminer species (Diptera: Agromyzidae) of economic importance in neotropical regions such as Amauromyza maculosa (Malloch) in Lacttuca sp. and Chrysantemum sp., Cerodontha dorsalis (Loew) in cereals; Japanagromyza Sasakawa sp. in Phaseolus sp. and Brassica sp., Liriomyza brassicae (Riley) in Brassicaceae family; L. huidobrensis (Blanchard), L. sativae Blanchard, and L. trifolii (Burgess) in vegetables; L. graminivora Hering in maize (Zea mays L.); and L. quadrata Malloch in potato (Solanum tuberosum L.) and tomato (Lycopersicum esculentum Mill.). C. flacilla has also been recovered from leafminers of minor importance such as Calycomyza achalensis Valladares, C. brewereae Valladares, C. cruciata Valladares, C. humeralis (Roser), C. longicauda Blanchard, C. malvae (Burgess), C. mikaniae Spencer, C. platyptera (Thomson), C. verbenivora Spencer, Chromatomyia platensis (Brethes), Haplopeodes gomphrenae Valladares, H. cordobensis Valladares, H. lycivora Valladares, Haplopeodes Steyskal spp., Liriomyza cesalpiniae Valladares, L. commelinae Frost, L. microglossae Spencer, L. near to ortizi, L. sabaziae Spencer, L. schmidti (Aldrich), L. spencerella Valladares, L. thalictrivora Spencer, Melanagromyza virens (Loew), Ophiomya camarae Spencer, Phytomyza crassiseta Zetterstedt, P. pampeana Blanchard, and P. williamson Blanchard.
Morphology
Egg
Eggs are 0.0733 x 0.0933 mm in size and are monoembryonic, transparent, and oval with rounded edges and with a glabrous surface. A thick and smooth chorion is present.
Larva
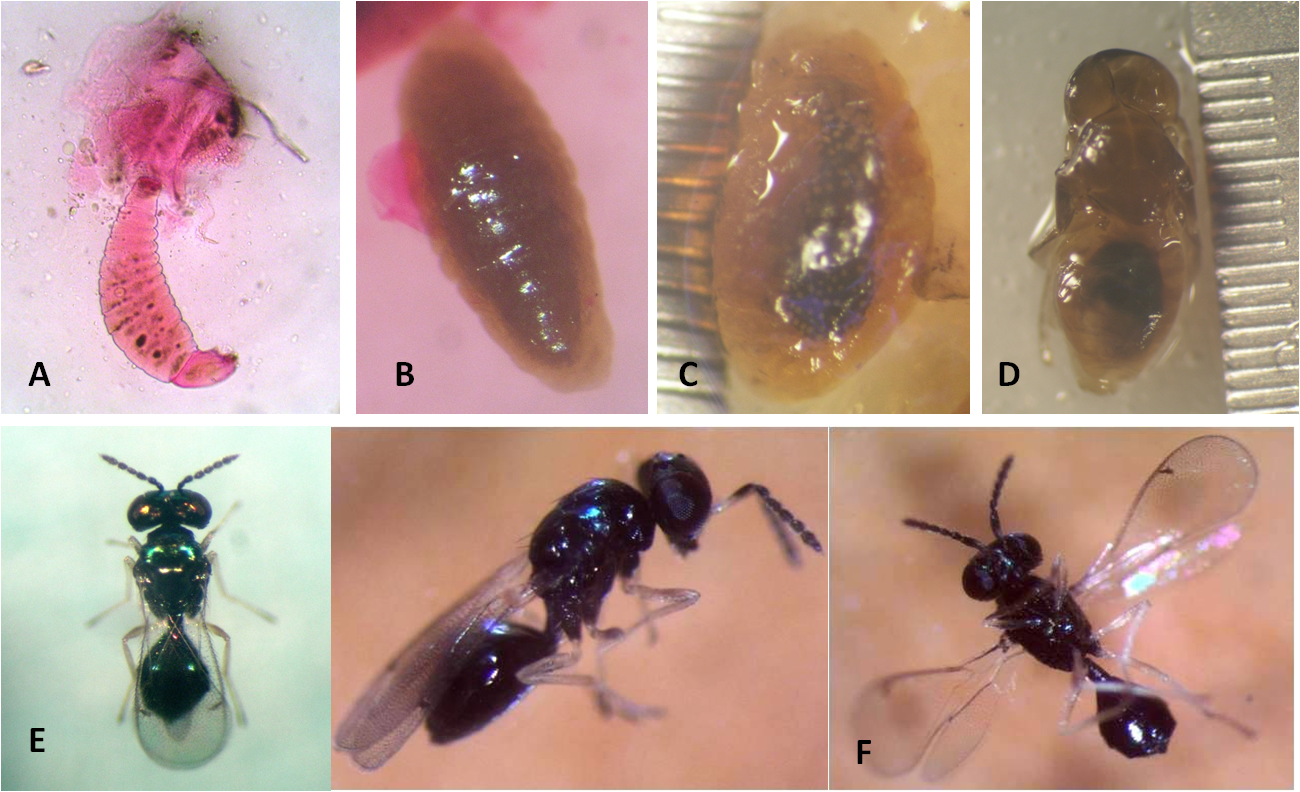
Three larval instars are described. The first instar is hymenopteriform (4.4 x 1.4 mm) with 12 segments, including the conical cephalic capsule with triangular mandibles that are the most sclerotic part of the body (Photo 1A). Second (11.1 x 4.5 mm) (Photo 1B) and third (10.9 x 4.3 mm) (Photo 1C) instars are vermiform.
Pupa
The pupa is exarata, transparent white to very dark brown (Photo 1D).
Adult
Female. A dark body 1.1–2.1 mm long with a blue-to-dark metallic green sheen (Photo 1E). The head has 5-segmented antennae, inserted on the ocellar suture, scapus pale with apical part darkened or completely dark; remaining antennae are dark. Abdomen is rhomboid and angular with ovipositor retractable, ventral middle position, and oriented at the front. All coxae are dark and metallic; fore and mid-coxae with weak and hind coax with strong reticulation. Remaining parts of legs are pale with 4th tarsal segment on all legs infuscate. Petiolus: About twice as long as wide, with protruding forecorners; upper surface reticulate to striate (occasionally punctulate). Male. Very similar to the female but with a wider and thicker scapus; reaching front ocellus (Photo 1F).
Biology
Parasitism
C.flacilla is a monoembrionic and koinobiont larval endoparasitoid of leafminer flies (Agromyzidae). The behavior of adult emergence, oviposition, and mating is typical of parasitic Chalcidoidea (see section 5.1.4). It usually places an egg by posture, although up to 6 eggs per host larva have been seen. Only one larva develops per host. The sex ratio gives a predominance of females (>80%) over males. Unfertilized eggs produce only females (Thelytoky parthenogenesis). Host-feeding behavior is observed in female adults.
Temperature-dependent development
Mean immature developmental period (egg to adult, using L. huidobrensis as the host) tends to decrease with increasing temperatures of 10°–30°C, with averages of 62 and 14 days, respectively (see Annex 7.4.6). The lower theoretical threshold of development was 3.2°C for egg-larvae and 5.7°C for pupae. Mortality of egg-larvae was around 10–15% between 15°C and 25°C. For pupae, mortality was lowest at 20°C (25%) and increased sharply at higher (64% at 30°C) and lower (80% at 10°C) temperatures. Lifespan decreased with increasing temperature, reaching a maximum of 30 and 26 days at 10°C and a minimum of 6 and 4 days at 30ºC for females and males, respectively. Oviposition of C. flacilla is significantly affected by temperature, showing a high variability in the progeny production. The lowest oviposition was observed at 10°C (0.7 offspring/female) and 30°C (1.1 offspring/ female) and the highest at 25°C (43.3 offspring/female). The sex ratio is highly affected by temperature, with a predominance of females at all temperatures.
The functions that had been established to describe the development time and rate, mortality, and reproduction were compiled into an overall phenology model and the following life-table parameters were calculated (see Annex 7.4.6). The intrinsic rate of increase (rm) and the finite rate of increase (λ) had positive values, 17°–29.5°C, indicating a population growth among these temperatures. A peak at 24.5°C (r: 0.085; λ: 1.089) was observed. At this temperature range doubling time (Dt) was shortest at 8.1 days. The mean generation time (T) decreased with temperature and was shortest at 29.5°C, with 20 days from egg to egg. The gross and net (Ro) reproduction rates were highest at 23°C (24 female offspring/female) and 22°C (12 female offspring/female), respectively. The optimum temperature for overall population growth ranged 23°C–26oC. C. flacilla presents a higher intrinsic and finite rate of increase than L. huidobrensis (see section 4.3.1), showing its potential as effective biological control agent of this pest.
Economic impact in pest control
Experimental studies of inundative/augmentative biological control against L. huidobrensis were carried out in the Carchi province, Ecuador, at 2,930 masl at a mean temperature of 12ºC. Releases of Chrysocharis sp. were made in faba bean (Vicia faba L.) (500 adults/plot at 40, 50, and 60 days after planting) and potato (500 adults/plot at 60, 80, and 100 days after planting). Parasitism by Chrysocharis sp. was 32% and 40% in potato and faba bean, respectively. In contrast, in untreated controls a parasitism of only 12.3% was found. C. flacilla attacks 33 of 45 available host species in different habitats in Cordoba, Argentina. Considering host-plant taxonomy (number of plant species in which leafminers were parasitized), leafminer hosts were parasitized by C. flacilla in 66 of 106 available plant species, of which the family Asteraceae was the most representative with 18 plant species. At the Peruvian coast, C. flacilla was one of the most important parasitoids of the L. huidobrensis parasitoid complex. In the southern coast, C. flacilla was the dominant species, representing 30–55% of the total parasitism in commercial potato fields. Also, C. flacilla has been recorded as dominant parasitoid species of L. huidobrensis (85%) in the highlands of Jujuy, Argentina. In classical biological control programs, C. flacilla was imported from Peru and introduced to Kenya and released as an exotic parasitoid. This was done in combination with the pteromalid Halticoptera arduine (Walker) and the braconid Phaedrotoma scabriventris Nixon (see sections 5.1.4 and 5.1.5) for controlling the invasive leafminer flies L. huidobrensis, L. sativae, and L. trifolii (see sections 4.3.1–4.3.3). C. flacilla was released at three different vegetable production altitudes in 2013. Field monitoring in 2014 indicated that the parasitoid successfully established at all the release sites; specific parasitism is still low. No spread assessment of the parasitoid has been conducted.
Geographical distribution
Possible regions of origin: Neotropics: Argentina, Chile, Colombia, Ecuador, El Salvador, Mexico, Peru, and Uruguay
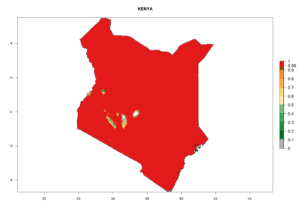
Introduced and established: In Kenya for the classical biocontrol of Liriomyza huidobrensis, L. sativae, and L. trifolii (Fig. 1).
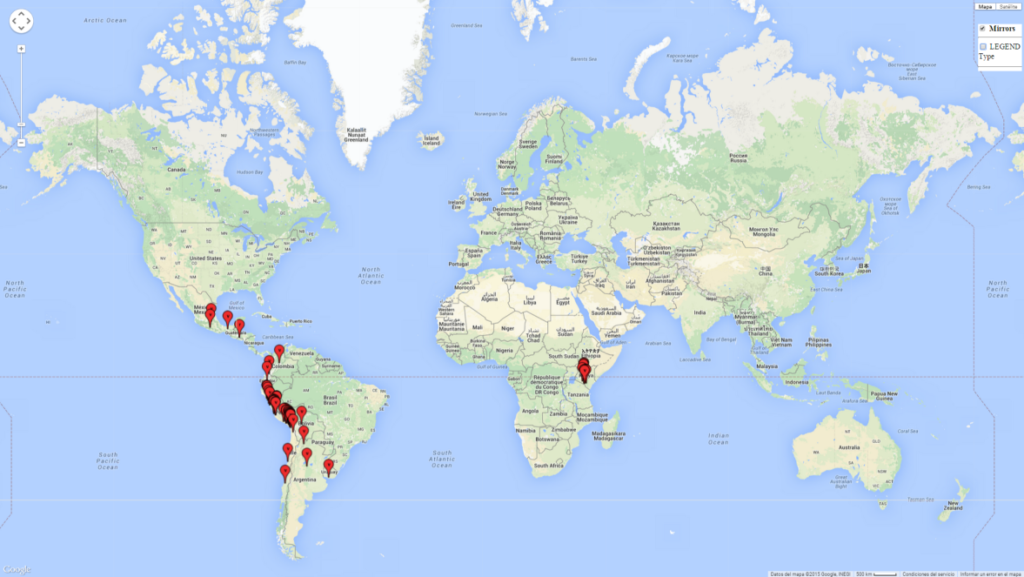
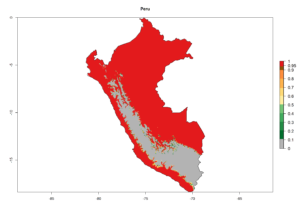
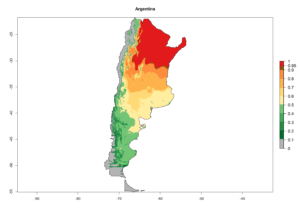
C. flacilla is adapted to wide ecological amplitude from the coastal region of Peru and Chile to the highlands of Peru at 4,045 masl. In Argentina it is an important parasitoid of Agromyzidae species in Cordoba, central Argentina (800 masl), with an average annual temperature of 16ºC (max. 24°C, min. 9°C). It also occurs in the highlands of Argentina, which is characterized by a warm climate and an average temperature of 18°–20°C (max. 30°C, min. 4°C). In Peru, C. flacilla was found along the coast from Lambayeque to Tacna, but with a higher abundance in the southern regions, which are characterized by a semi-warm and very dry climate (desert, arid, and subtropical) with a mean temperature of 19°C. This species was also found in the central highlands of Peru (3,250–4,042 masl), with an annual average maximum temperature of 23°C and a minimum of 4°C.
Potential establishment and efficiency under current and future climates
Changes in global establishment and distribution
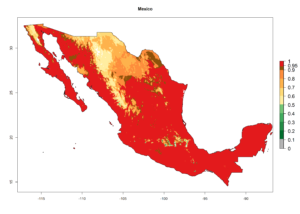
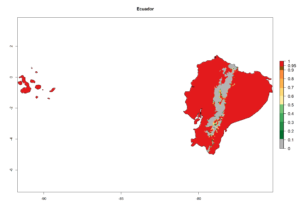
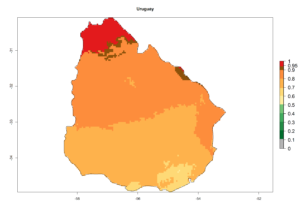
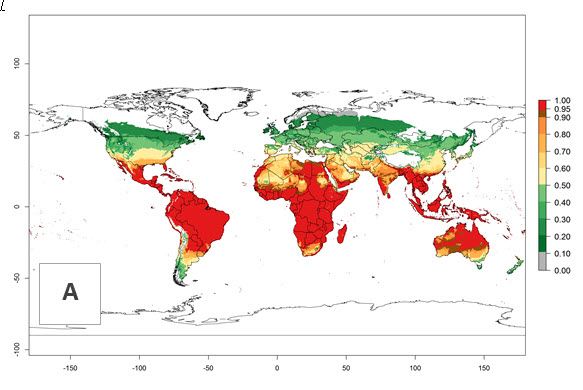
An establishment index (EI)=1 indicates survival of the parasitoid throughout the year (i.e., the likelihood of long-term establishment for classical biological control is very high in these regions). Current distribution of C. flacilla is the assumed regions of origin such as Mexico (Guerrero, Mexico D.F., Chiapas); Ecuador (El Carchi); and Peru (lowland and highland valleys), which are well reflected by an establishment index (EI)>0.95. However, C. flacilla has also been established in regions with an EI>0.7–0.9 (light and dark orange zones) as in Argentina (Cordoba) and with an EI>0.6–0.7 (dark yellow zones) as in Uruguay (Montevideo) (Fig. 2; compare with Fig. 1).
 |
 |
 |
 |
 |
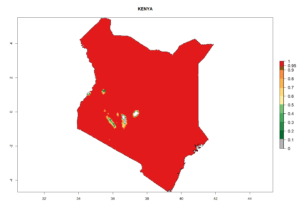 |
Figure 2. EI of Chrysocharis flacilla in countries where establishment has been reported, according to model predictions for the year 2000. An EI>0.6 indicates regions with permanent establishment.
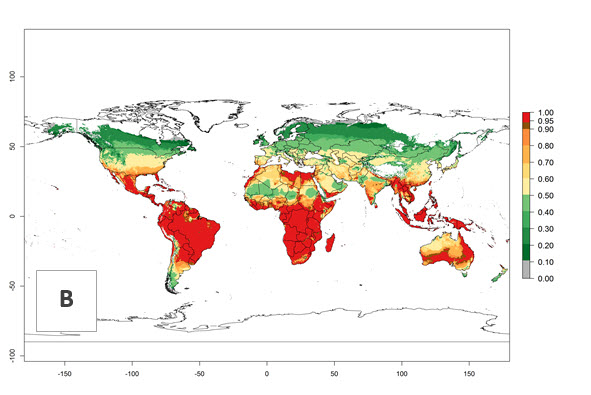
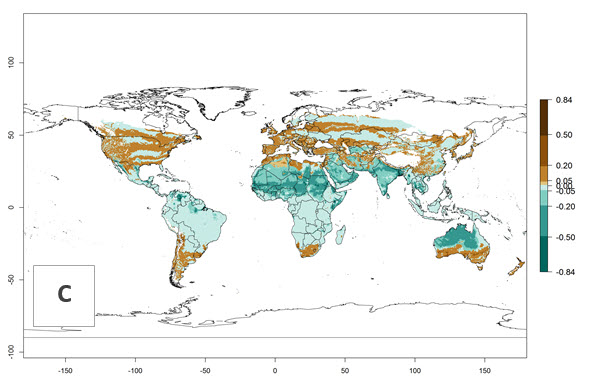
Global predictions for 2000 indicate a high potential of establishment of C. flacilla (EI>0.95) in tropical zones of Africa, America, Asia, and Oceania (Fig. 2A), where the leafminer flies also have a high ERI. In most of the subtropical regions an EI>0.6–0.9 is predicted. For the 2050 climate change scenario, C. flacilla increases or mainly maintains a high establishment potential (EI>0.95) in subtropical regions. A slight range expansion is projected to more temperate regions of southern South America, USA, Asia, Europe, and Oceania, with an EI>0.6 (Fig. 2B, C). By contrast, an insignificant decrease is estimated in the establishment of C. flacilla in Central and South America; Central, East, and Southern Africa, and Southeast Asia.
 |
 |
 |
|
Figure 3. Changes in establishment and potential distribution worldwide of Chrysocharis flacilla according to model predictions, using the EI for the years 2000 (A) and 2050 (B), and changes of the EI between 2000 and 2050 (C). An EI>0.6 indicates regions with potential permanent establishment.
Changes in global abundance
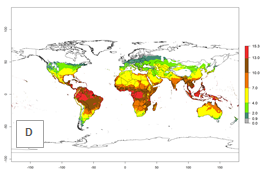
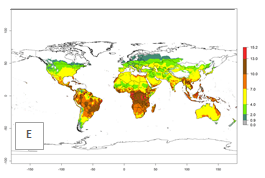
The generation index (GI) for the year 2000 estimates the development of 13–21 generations per year for tropical regions and 7–16 generations per year for most subtropical regions (Fig. 4A). The number of generations in countries where C. flacilla is established today are >13 per year in Colombia, Ecuador, Mexico (Chiapas, Guerrero), and El Salvador; 10–16 per year at the northern coast of Peru and Cordoba, Argentina; 7–10 per year at the central and southern coast of Peru and Uruguay; and 4–10 per year at the coast of Chile, in the highlands of Peru, Ecuador, and Colombia, and in Mexico (Mexico D.F.). The GI change indicates that a potential increase of 1–3 generations per year of C. flacilla can be expected in most tropical and subtropical regions where L. huidobrensis is established. For temperate zones up to 1 generation per year is predicted. In some tropical zones of Central and South America as well as Africa, an increase of 3–5 generations per year can be expected according to the predictions (Fig. 4B, C).
Global maps of the activity index (AI) for the year 2000 estimate the highest activity (AI>10) of C. flacilla in tropical zones of Central and South America, the Caribbean, Africa (central and eastern regions), and Asia (southeast region) (Fig. 4D). Potential population growth in countries where C. flacilla is established today ranges AI>13–15.3 in lowlands of Colombia, Ecuador, and El Salvador, and an AI>4–7 at the northern coast of Chile and the highlands of Peru and Uruguay. Predictions of changes for the 2050 temperature scenario estimate an increase of the potential population growth by a factor of up to 10 in Central (Mexico) and South America (highland Andes, southern Brazil, Argentina); East and Southern Africa; and southern China (Fig. 4E, F). Also, a low increase by a factor of 0–2 is predicted for temperate zones. By contrast, a high decrease by a factor from -2 to -12 is predicted in most tropical regions and up to -2 for subtropical regions.
| GI | AI | |
| 2000 | 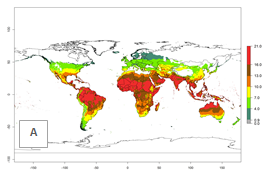 |
 |
| 2050 | 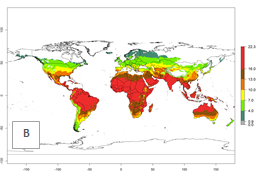 |
 |
| Index change (2000–2050) | 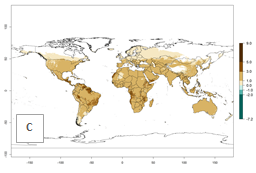 |
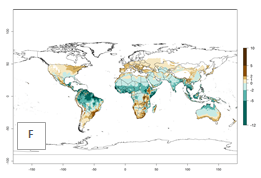 |
Figure 4. Changes in abundance (GI, number of generations/year) and activity (AI, potential population growth) of Chrysocharis flacilla worldwide according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
Changes in regional establishment and distribution in Africa
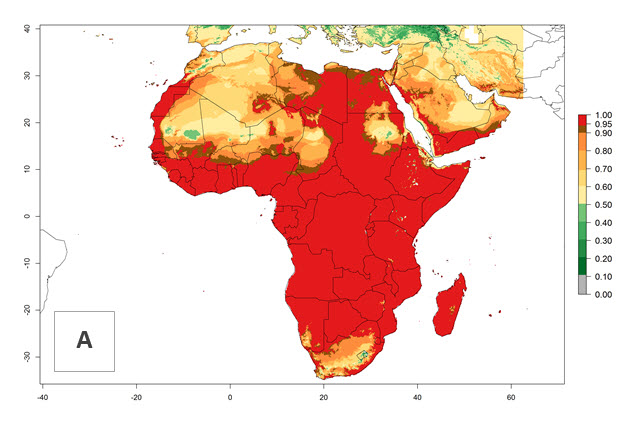
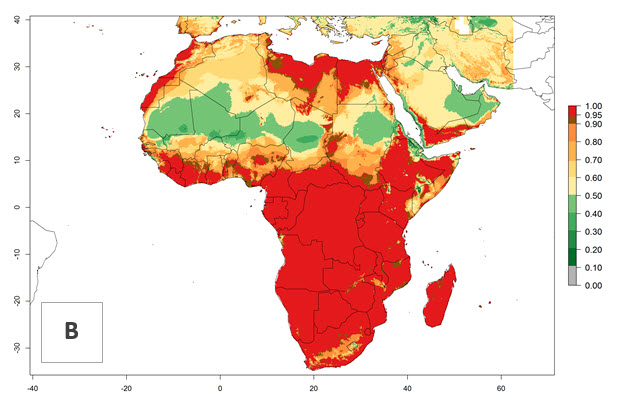
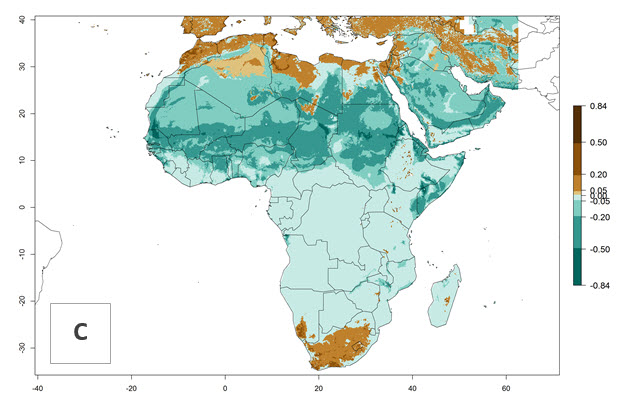
C. flacilla was introduced into areas of Kenya with a high establishment index (EI>0.95), with subsequent successful establishment. According to the mapping results, a successful establishment (EI>0.95) could also be expected in most countries of North, West, East, Central, and Southern Africa under the year 2000 temperature conditions (Fig. 5A). Owing to climate change, it can be expected that the establishment will potentially increase in South Africa and the Mediterranean region of North Africa (Fig. 5B). C. flacilla will maintain a high establishment (EI>0.95) by 2050 in most countries of East, Southern, and Central Africa; only a slight decrease in establishment (<-0.05) is predicted in these regions (Fig. 5B, C). The Sahara region will present unfavorable temperatures for C. flacilla development, where a considerable reduction in establishment (up to -0.5) is expected. At the same time, Liriomyza spp. will also have less potential to establish and its pest status will decrease (see section 4.3.1).
 |
 |
 |
|
Figure 5. Changes in establishment and potential distribution of Chrysocharis flacilla in Africa according to model predictions, using the EI for the years 2000 (A) and 2050 (B) and changes of the EI between 2000 and 2050 (C). An EI>0.6 indicates regions with permanent establishment
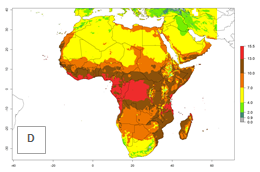
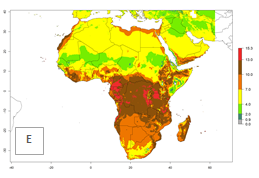
Changes in regional abundance in Africa
The GI for the year 2000 estimates 13–21 generations per year for tropical regions of Africa. In most of the subtropical regions, 10–16 generations per year are predicted (Fig. 6A). Predictions for the 2050 climate change scenario estimate an increase of 1–3 generations per year in almost all of Africa (Fig. 6B, C). For some areas of countries in North (Occidental Sahara); Central (Congo, Angola); and West (Ethiopia, Tanzania, Malawi, Mozambique) Africa, an increase of 3–4 generations per year is predicted. The GI is strongly correlated with the AI. The activity is expected to increase in some areas of Africa, especially in East (Ethiopia, Uganda, Kenya, Tanzania, Rwanda, Malawi, Madagascar, Zambia); Central (Angola); and Southern Africa (South Africa, Namibia, Botswana, Zimbabwe), where a high C. flacilla activity of AI>10 is projected (Fig. 6E, F).
| GI | AI | |
| 2000 | 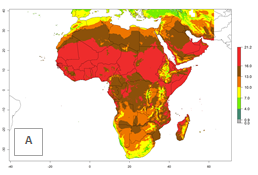 |
 |
| 2050 | 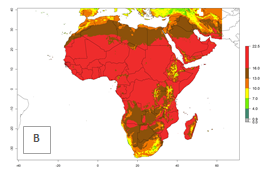 |
 |
| Index change (2000 – 2050) | 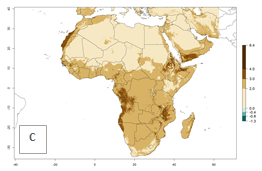 |
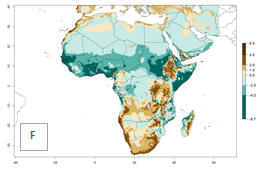 |
Figure 6. Changes in abundance (GI, number of generations/year) and activity (AI, potential population growth) of Chrysocharis flacilla in Africa according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
Potential release areas in Africa
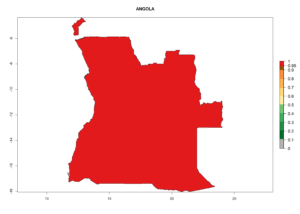
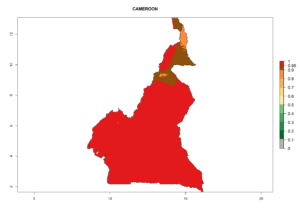
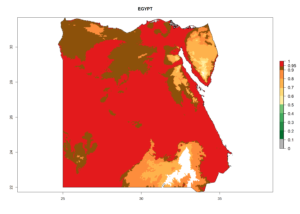
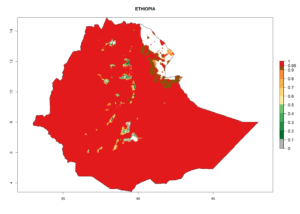
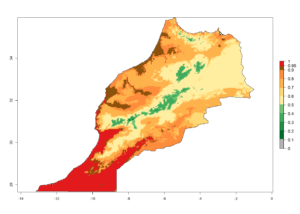
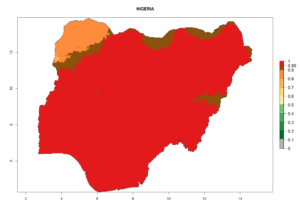
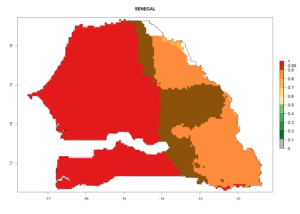
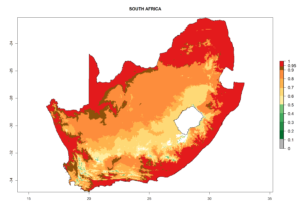
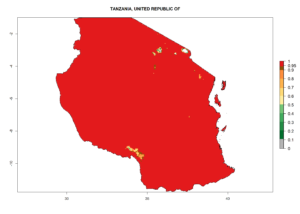
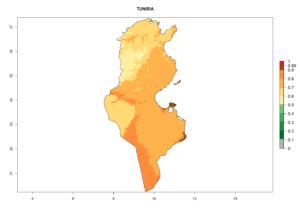
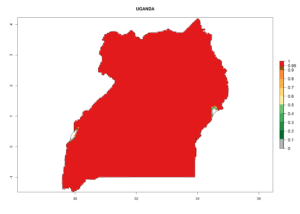
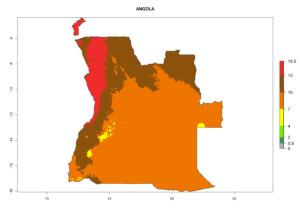
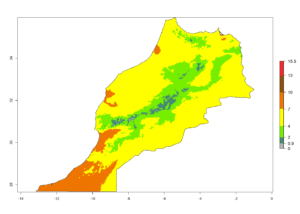
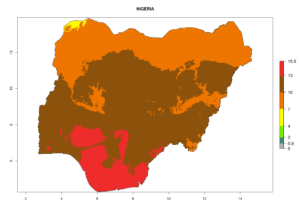
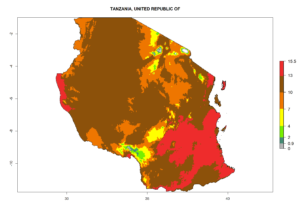
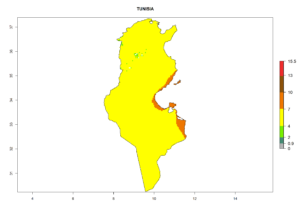
Considering the highest EI, GI, and AI of L. huidobrensis in different countries of Africa (section 4.3.1), potential releases of C. flacilla under the present climate can be mainly considered in the countries of West (Senegal, Nigeria, Cameroon); East (Ethiopia, Kenya, Uganda, Rwanda, Tanzania, Madagascar); and Central (Angola) Africa. In these countries the likelihood of establishment of C. flacilla is expected to be very high (EI>0.95) and is associated with a GI>10 (i.e., more than 10 generations per year) and an AI>7 (Fig. 7). C. flacilla could also be considered for releases in some areas of the Mediterranean region (Morocco, Tunisia, Egypt) and in Southern Africa (South Africa), where the likelihood of establishment is still high (EI>0.6) and is associated with a GI>4–10 (i.e., 4–10 generations per year) and a potential population growth of AI>2–7.
| EI | GI | AI |
| a) Angola | 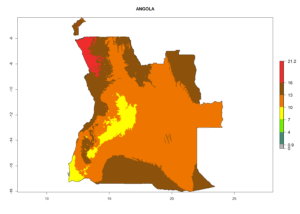 |
 |
| b) Cameroon | 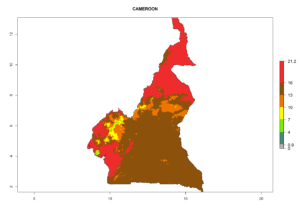 |
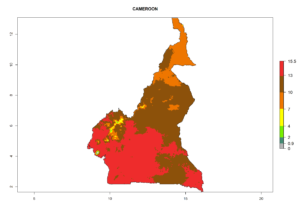 |
| c) Egypt | 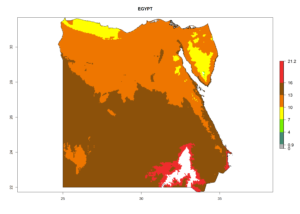 |
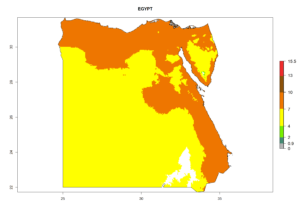 |
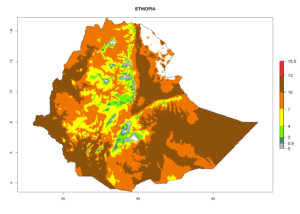
| d) Ethiopia | 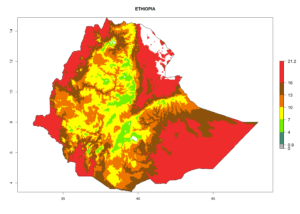 |
 |
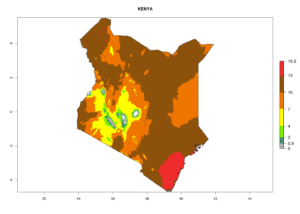
| e) Kenya | 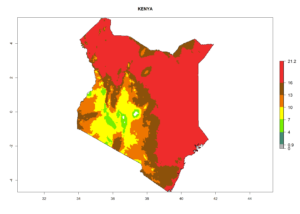 |
 |
| f) Morocco | 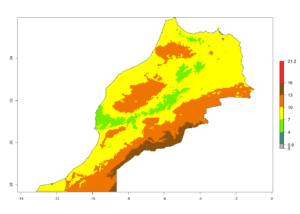 |
 |
| g) Nigeria | 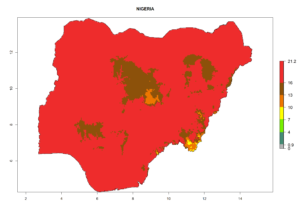 |
 |
| h) Senegal | 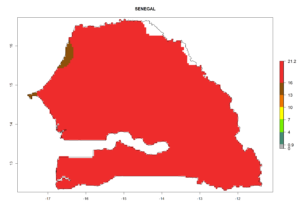 |
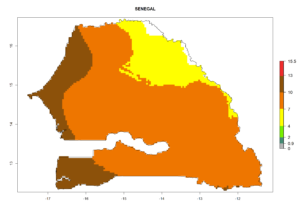 |
| i) South Africa | 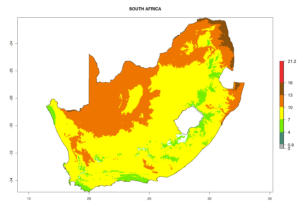 |
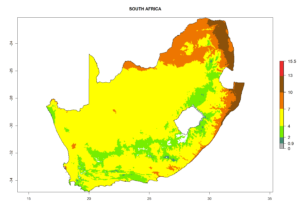 |
| j) Tanzania | 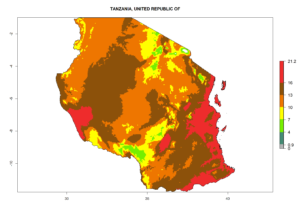 |
 |
| k) Tunisia | 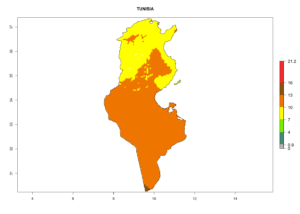 |
 |
| l) Uganda | 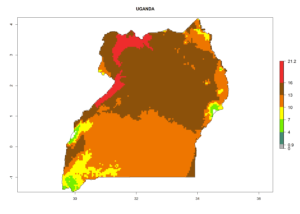 |
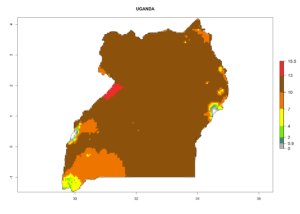 |
Figure 7. EI, abundance (GI, number of generations/year), and activity (AI, potential population growth) of Chrysocharis flacilla in select African countries according to model predictions for the year 2000. An EI>0.6 indicates regions with permanent establishment.
Risks to non-targets
No risks are reported. C. flacilla is a parasitoid of Agromyzidae leafminer flies with a wide host range. This also includes species that are non-agricultural pests. In the region of evolution the parasitoid lives in a natural balance and equilibrium with any kind of leafminer host, where (to our knowledge) it has not caused the extinction of any species. After its release in new agro-ecosystems it is hoped that the species will adapt and naturalize in its new environment in order to achieve high impact by reducing the infestation of agricultural Agromyzidae pests like L. huidobrensis. In addition to parasitism of target pests, the introduced parasitoid may also parasitize local leafminer species occurring in the natural environment. This, however, is unlikely to cause an extinction of a species.
Further reading
Arce de Hamity, M., and L. Neder de Román. 1984. Detection of injurious and beneficial insects in crops of Vicia faba in upland areas. Revista de la Sociedad Entomológica Argentina 43(1/4): 7–11.
Boucek, Z. 1977. Descriptions of two new species of Neotropical Eulophidae (Hymenoptera) of economic interest, with taxonomic notes on related species and genera. Bulletin of Entomological Research 67(1): 4.
Chulde, R., and P. Gallegos. 2002. Control biológico de la mosca minadora Liryomyza huidobrensis con dos parasitoides Chrysocharis sp. y Diglyphus sp. en papa, Solanum tuberosum. Instituto Nacional Autónomo de Investigaciones Agropecuarias. Universidad Central de Ecuador.
Costa-Lima, T.C. 2011. Bioecologia y competencia de dos especies de parasitoides neotropicales (Hymenoptera: Braconidae y Eulophidae) de Liriomyza sativae Blanchard, 1938 (Diptera: Agromyzidae). PhD diss., Universidad de Sao Paulo, Sao Paulo, Brazil.
De Santis, L. 1983. Catálogo de los Himenopteros Calcidoideos de América al Sur de los Estados Unidos. Primer Suplemento. Revista Peruana de Entomología 24(1): 22.
Hansson, C. 1987. Revision of the New World species of Chrysocaris Forster (Hymenoptera: Eulophidae). Entomologica Scandinavica 29: 1–86.
Mujica, N. 2007. Malezas hospederas de moscas minadoras (Diptera: Agromyzidae) y sus parasitoides en el ecosistema de papa en La Molina. MSc thesis, Universidad Nacional Agraria La Molina, Lima, Peru.
Mujica, N., and J. Kroschel. 2011. Leafminer fly (Diptera: Agromyzidae) occurrence, distribution, and parasitoid associations in field and vegetable crops along the Peruvian coast. Environmental Entomology 40(2): 217–230.
Mujica, N., C. Prudencio, C. Valencia, L. Rodriguez, K. Fiaboe, and J. Kroschel. 2013. Potential of hymenopteran parasitoids for classical biocontrol of leafminer flies (Diptera: Agromyzidae). In: 4th International Symposium on Biological Control of Arthropods, March 4–8, Pucon, Chile.
Neder de Roman, L., and M. Arce de Hamity. 1984. Review of and new contributions to knowledge of the bioecology of Liriomyza huidobrensis (Diptera: Agromyzidae). Acta Zoologica Lilloana 37(2): 295–301.
Ramirez, L. 2008. Efecto de la temperatura sobre el desarrollo, longevidad y fecundidad de Chrysocharis flacilla Walker (Hym.: Eulophidae) parasitoide de Liriomyza huidobrensis (Blanchard) (Dip.: Agromyzidae). BSc thesis, National Agricultural University La Molina, Lima, Peru.
Salvo, A., and G. Valladares. 1997. An analysis of leafminer and plant host ranges of three Chrysocharis species (Hym.: Eulophidae) from Argentina. Entomophaga 42(3): 417–426.
Salvo, A., and G. Valladares. 2001. Community dynamics of leafminers (Diptera: Agromyzidae) and their parasitoids (Hymenoptera) in a natural habitat from Central Argentina. Acta Oecologica 22: 301–309.
Sanchez, V.G., and I. Redolfi de Huiza. 1985. Parasitoids of Liriomyza huidobrensis and Scrobipalpula absoluta in potatoes cultivated in Lima (Peru). Revista Peruana de Entomología 28: 81–84.