4.2.2 Sweetpotato Pests / Sweetpotato weevil, Cylas brunneus (Fabricius)![]()
Synonyms: Cylas angustatus (Labram and Imhoff 1838; Labrouise 1842; Pierce 1940; Marshall 1953)
Brentus brunneus (Olivier 1790)
Taxonomic position: Insecta, Coleoptera, Brentidae
Authors: P. Musana, J.S. Okonya, N. Mujica, P. Carhuapoma, & J. Kroschel
Common name
Sweetpotato weevil (English), Liberian sweetpotato borer (English), Charançon de la patate douce (French)
Hosts
Sweetpotato (Ipomoea batatas (L.) Lam), Morning glory (I. I. eriocarpa R. Br.), Water spinach (I. aquatica Forssk)
Detection and identification
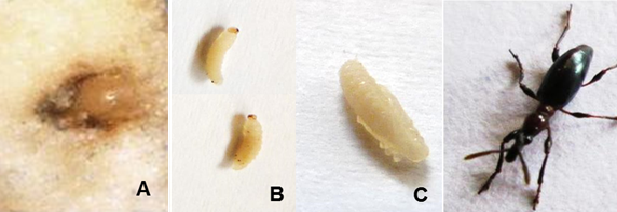
Adults and larvae of Cylas brunneus damage both sweetpotato foliage and storage roots through their feeding activity. Adults mainly feed on the external surface of roots, causing round feeding punctures; they also feed on leaves, which causes irregular to round punctures. In stems, larvae burrow and feed, causing malformation, thickening, and cracking of the affected vines that reduce size and number of storage roots. The first signs of C. brunneus injury are mostly observed at or near the crown of roots exposed to the surface. Larvae feed inside the root and deposit frass in the feeding galleries or tunnels, thus reducing root quality and yield (Photo 1A, B). The feeding activity of the larvae induces the production of bitter terpenoid substances in the infested roots and renders them unfit for human or animal consumption.
Photo 1. Sweetpotato root damage by sweetpotato weevil, Cylas brunneus: (A) adults and (B) larvae. Photos: Courtesy of CIP.
Morphology
Egg
Eggs are oval, light yellow, and measure 0.7 X 0.5 mm (Photo 2A). Newly laid eggs are translucent and soft with rugose surface. They turn creamy with small brown irregular specks just before hatching. Eggs are laid singly in small cavities created by the female mouthparts and very close to the skin of the sweetpotato root or stem. The female seals each egg within the oviposition cavity with a plug of fecal material, making it difficult to observe the egg.
Larva
Larvae are legless, curved, and creamy white with a brown sub-globular head and reddish brown gut. The abdomen is sub-conical for the first and second instars and sub-cylindrical from third to fifth instars. Mature larvae measure 7–8 mm in length (Photo 2B).
Pupa
The pupa is white and 4–5 mm long (Photo 2C). Pupation occurs inside the root in a small chamber prepared by the final instar larva.
Adult
Normally, adults emerge from the pupation sites by chewing a hole through the exterior of the plant tissue. But they sometimes remain inside the stem or root for a few days until they attain full coloration. Most enclosed adults of C. brunneus reach their final coloration by changing from white to brown; others turn completely black like C. puncticollis. C. brunneus adults are small (about 5–7 mm long), smooth and hard-bodied, with a black head, dark red thorax, black elytra, and light-brown abdomen resembling C. formicarius (Photo 2D). Although external differences between adult males and females are slight, with practice the sexes can be distinguished without magnification. The distal antennal segment of the males is filiform whereas those of the females are club-shaped.
Biology
Both sexes are apparently nocturnal and functionally winged. Males are more agile than females. When an adult C. brunneus is disturbed, it feigns death. Females feed for a day or more before becoming sexually active, but commence oviposition shortly after mating. The female adult C. brunneus produces a pheromone that attracts the male for mating, which is normally done at night when male weevils are more active searching for females. Although the females mate at night, they feed and lay eggs during the day.
Temperature-dependent development
Development of all C. brunneus live stages is possible at 17.5°–32°C (see Annex 7.3.5). At 20°C, the median immature development time was 9, 41, and 9 days for egg, larvae, and pupae. Development time decreased with rising temperatures and was, at 30°C, 3, 20, and 4 days for egg, larvae, and pupae. The upper threshold of development was 35°C for eggs and 32°C for larvae and pupae; the lower threshold was 17.5°C for all immature stages. Lifespan of adults decreased from 147 days at 25°C to about 66 days at 30°C. Mortality of all immature stages showed a similar temperature response: lowest at temperatures of 25°C (i.e., 8% for egg, 20% for larva, 4% for pupa) and highest at various temperatures (50% for egg at 35°C, 82% at 17.5°C, 58% at 32°C). Oviposition peaked at 25°C with 94 eggs laid/female; lowest oviposition (11 eggs) was registered at 17.5°C.
Life-table parameters of C. brunneus were estimated using various sub-models in an overall deterministic phenology model (see Annex 7.3.5). The intrinsic rate of increase (rm) and the finite rate of increase (λ) indicate a population growth between 20°C and 32°C, with peaks between 20°C and 30°C, where 1.06% of the population growth per day was estimated. At this temperature range, doubling time (Dt) was shortest with 10 days. The mean generation time (T) decreased with temperature and was shortest at 30°C, with around 48 days from egg to egg. The gross reproduction rate was highest at 22°–30°C, with about 59 female offspring/female. The net reproduction rate (Ro) was highest at 20°–30°C, with around 24 female offspring/female.
Means of movement and dispersal
C. brunneus fly occasionally and for only short distances, from 20 to 80 m. Adults can therefore move from infested host plants to neighboring clean fields. Human activity, however, remains key in the spread of weevils, particularly through transporting of storage roots and infested planting material.
Economic impact
C. brunneus is one of the most important insect pests of sweetpotato in Africa. Yet there is little published information on the exact economic loss of C. brunneus alone since it always co-exists in the field with C. punticollis. Sweetpotato leaf-feeding by the adult reduces the photosynthesis activity of the plant, stem injuries can lead to poor crop establishment, and root injuries by adult and larval activities reduce root yield and quality. Larval feeding induces terpenoid production in the roots and renders the infested roots unfit for consumption. Under field conditions, the two species of African sweetpotato weevils, C. brunneus and C. puncticollis, have been reported to cause yield loss of up to 80–100% in Uganda, >50% in Tanzania, and 50–90% in Kenya. Species-specific studies are needed to ascertain the exact root yield losses due to C. brunneus.
Geographical distribution
C. brunneus is restricted and endemic to Africa. It has been recorded in nine sweetpotato-producing countries in Africa (Fig. 1). Studies are needed to ascertain the exact distribution of C. brunneus in Africa.
| Africa | Uganda, Kenya, Rwanda, Burundi, Nigeria, Ghana, Ivory Coast, Sierra Leone, Togo |
Phytosanitary risks
C.brunneus is primarily a tropical and warm-temperate species that has been found up to altitudes greater than 2,400 masl in tropical regions of Africa.
Risks mapping under current and future climates
Global Risks
Changes in establishment and future distribution
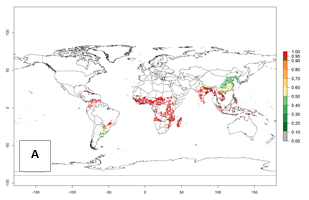
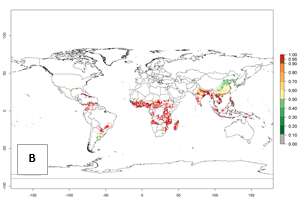
An establishment risk index (ERI>0.95) reflects well the current distribution of C. brunneus in Africa under the current climate of the year 2000 (Fig. 2A). C. brunneus has the potential to permanently establish outside Africa in most tropical areas of Central America (Honduras and Panama); South America (Colombia and Venezuela); the Caribbean (Cuba, Haiti, Dominican Republic); South Asia (India and Bangladesh); and Southeast Asia (Indonesia, Thailand, Vietnam, Malaysia, Philippines); and also in some subtropical areas of South America (Paraguay and southern Brazil) (compare with Fig. 1). In zones where the ERI drops below the maximum number of 1.0, the likelihood of establishment of C. brunneus is reduced.
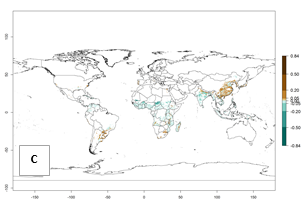
Global predictions for 2050 indicate a similar trend as predicted for C. puncticollis, and C. brunneus will remain a high risk pest (ERI>0.95) for most sweetpotato production areas in tropical regions (a slight decrease of <0.05 is observed) (Fig. 2B, C). A slight increase in the spread of C. brunneus but still with a very low establishment potential (ERI<0.6) is predicted for subtropical regions of Asia (northern India and southern China); Europe (Portugal); South America (northern Argentina, Uruguay, southern Brazil); and Southern Africa (South Africa).
 |
 |
 |
|
Figure 2. Changes in establishment and potential distribution of the sweetpotato weevil, Cylas brunneus, in sweetpotato production systems worldwide according to model predictions, using the ERI for the years 2000 (A) and 2050 (B), and changes of the ERI between 2000 and 2050 (C). An ERI>0.95 is associated with potential permanent establishment.
Changes in abundance
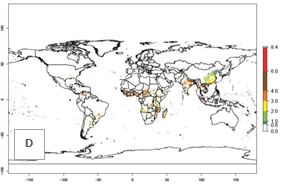
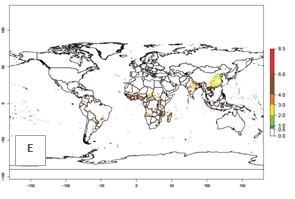
Under the current temperatures, mainly 4–6 generations per year can develop in most of West Africa, whereas fewer generations (2–4 per year) can develop in East and <4 generations per year in Southern Africa (Fig. 3A). Potentially, 4–6 generations can develop in most of tropical sweetpotato-producing areas outside Africa, including Southeast Asia; South Asia (India and Bangladesh); Central and South America (Venezuela and Colombia); and the Caribbean (Cuba and Haiti). In subtropical areas (southern Brazil, Uruguay, Paraguay, northern Argentina, southern China), 1–4 generations per year are predicted. In 2050, however, the number of generations per year for C. brunneus will be highest in West and Central Africa, with nearly 7 generations per year, followed by Southern Africa, with mostly 5–6 generations per year, and East Africa, with mostly 4–5 generations per year (Fig. 3B). The generation index (GI) change indicates that in most tropical regions of Africa, an increase of 0.5–1 generations can potentially be expected, as in West, Central, and East Africa (Fig. 3C).
Global maps for the activity index (AI) in the year 2000 estimate a high activity and spread potential of up to 108 in C. brunneus populations in tropical regions of Africa, Central and South America, and South and Southeast Asia (Fig. 3D). Predictions of changes for 2050 scenario show a slight increase in the potential growth of C. brunneus population in most of the tropical and subtropical countries where sweetpotato is grown (Fig. 3E, F).
| GI | AI | |
| 2000 | 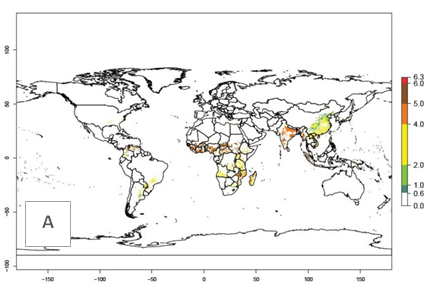 |
 |
| 2050 | 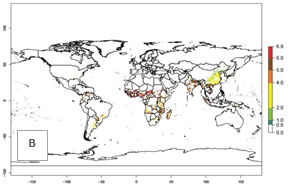 |
 |
| Index change (2000 – 2050) | 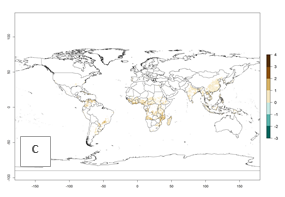 |
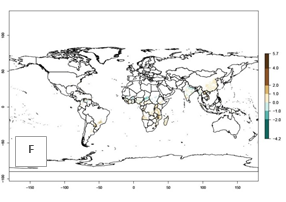 |
Figure 3. Changes in abundance (GI, damage potential) and activity (AI, potential population growth) of the sweetpotato weevil, Cylas brunneus, in sweetpotato production systems worldwide according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
Regional Risks for Africa
Changes in establishment and future distribution
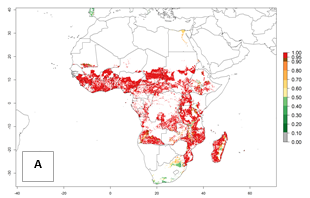
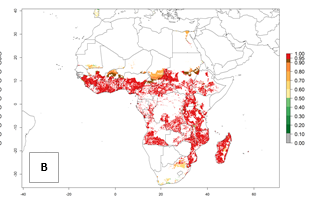
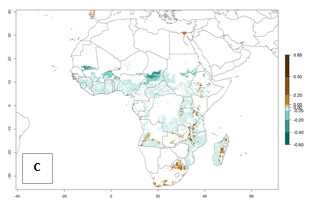
In Africa, C. brunneus occurs naturally in some countries in West (Nigeria, Ghana, Ivory Coast, Sierra Leone, Togo) and East (Uganda, Kenya, Rwanda, Burundi) Africa, with an ERI>0.95 (Fig. 4A). Suitable temperature conditions are present for the establishment in other countries such as Tanzania, Ethiopia, South Sudan, DR Congo, Central Africa Republic, Chad, Niger, Mozambique, Congo, Zambia, Angola, Madagascar, Cameroon, Liberia, Benin, Burkina Faso, Mauritania, Gabon, Malawi, Sudan, Guinea, and Madagascar (Fig. 4A). Global predictions for 2050 indicate that the high risk of establishment (ERI>0.95) will potentially remain in many parts of all tropical countries (Fig. 4B, C). A slight range expansion might be possible to East and some parts of Central Africa (Ethiopia) as well as to Southern Africa (Malawi, Angola, Zambia, northern South Africa, Madagascar), reaching a high establishment potential of the pest (ERI>0.8). A decrease (-0.2 to -0.5) in establishment is estimated for some areas of West Africa (Chad, Burkina Faso, Senegal).
 |
 |
 |
|
Figure 4. Changes in establishment and potential distribution of the sweetpotato weevil, Cylas brunneus, in African sweetpotato production systems according to model predictions, using the ERI for the years 2000 (A) and 2050 (B), and changes of the ERI between 2000 and 2050 (C). An ERI>0.95 is associated with potential permanent establishment.
Changes in abundance
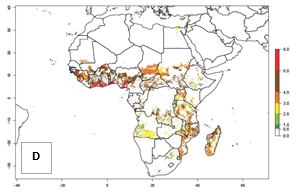
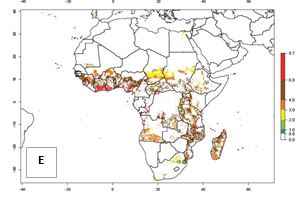
Under the current temperatures, mostly 4–6 generations per year can develop in most of West and Central Africa, whereas mostly fewer generations (1–4 per year) can develop in East and Southern Africa (Fig. 5A). The GI change indicates that in most tropical regions of Africa, an increase of 0.5–2 generations can potentially be expected (Fig. 5C).
| GI | AI | |
| 2000 | 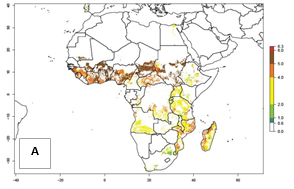 |
 |
| 2050 | 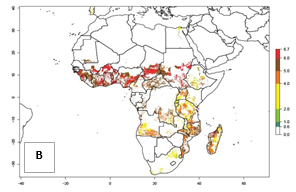 |
 |
| Index change (2000 – 2050) | 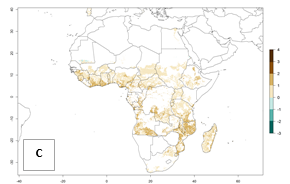 |
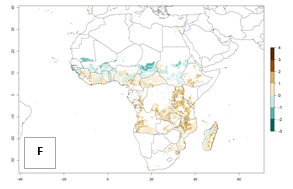 |
Figure 5. Changes in abundance (GI, damage potential) and activity (AI, potential population growth) of the sweetpotato weevil, Cylas brunneus, in African sweetpotato production systems according to model predictions using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
The AI in year 2000 estimates a high population increase of up to 108 in tropical regions of West and Central Africa. In Southern and East Africa, an increase of up to 106 in C. brunneus populations is estimated (Fig. 5D). Predictions of changes for 2050 scenario show a slight increase in the potential growth of C. brunneus population in most of the sub-Saharan African countries where sweetpotato is grown (Fig. 5E, F). Parts of some tropical countries in West Africa (Chad, Nigeria, Benin, Togo) will register a slight decrease in the potential population growth of C. brunneus by the year 2050 (Fig. 5E, F).
Country Risk Maps
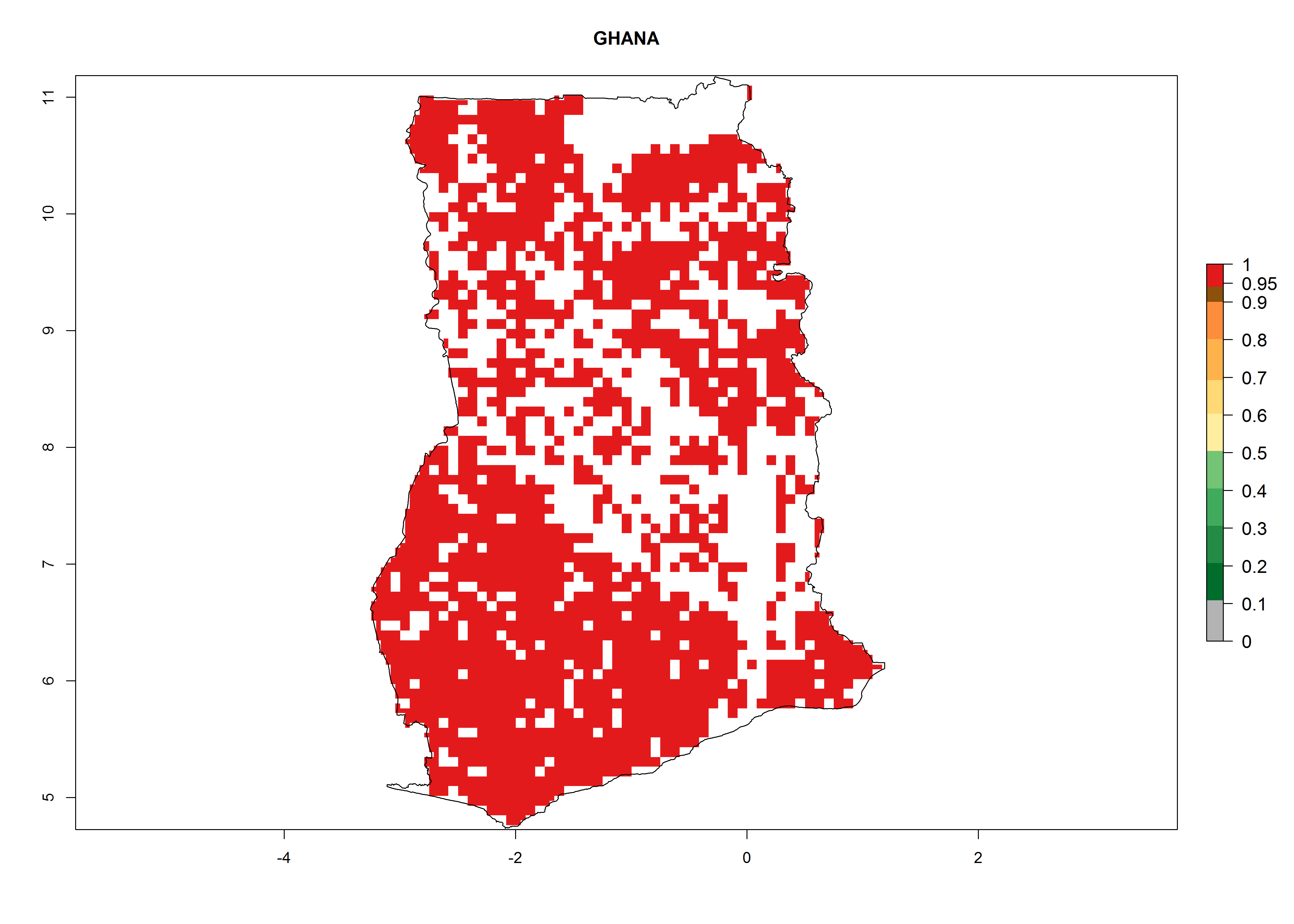
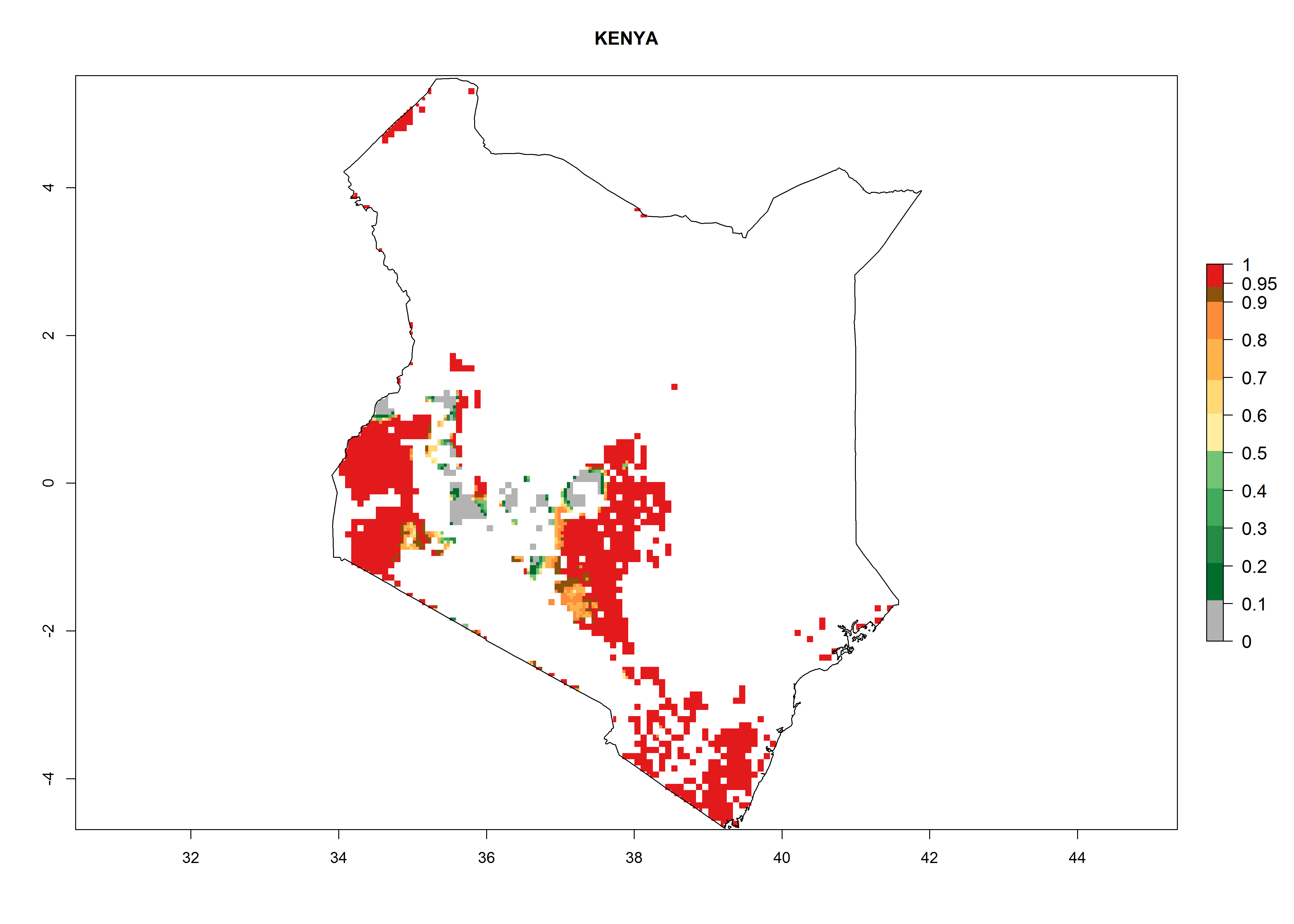
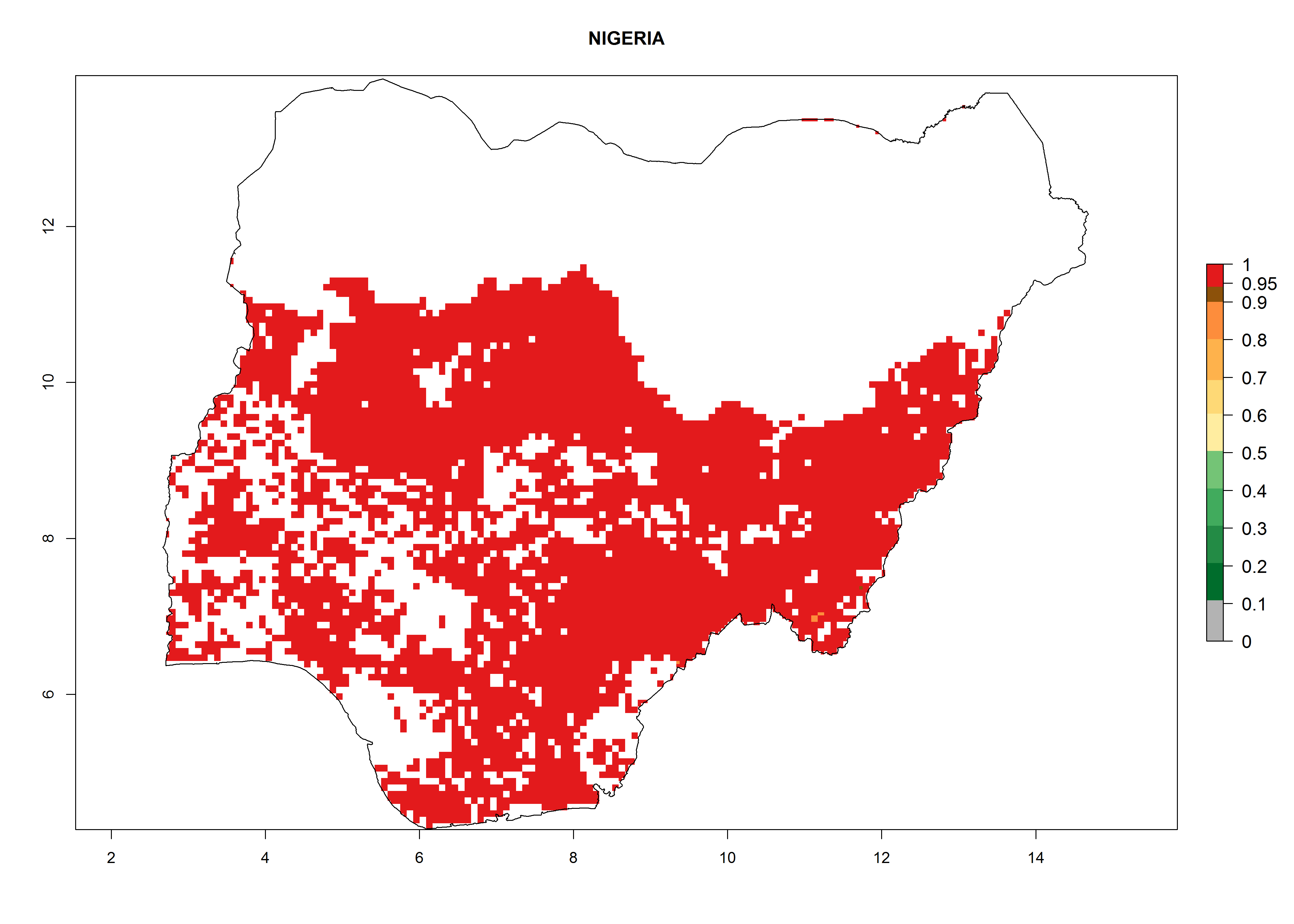
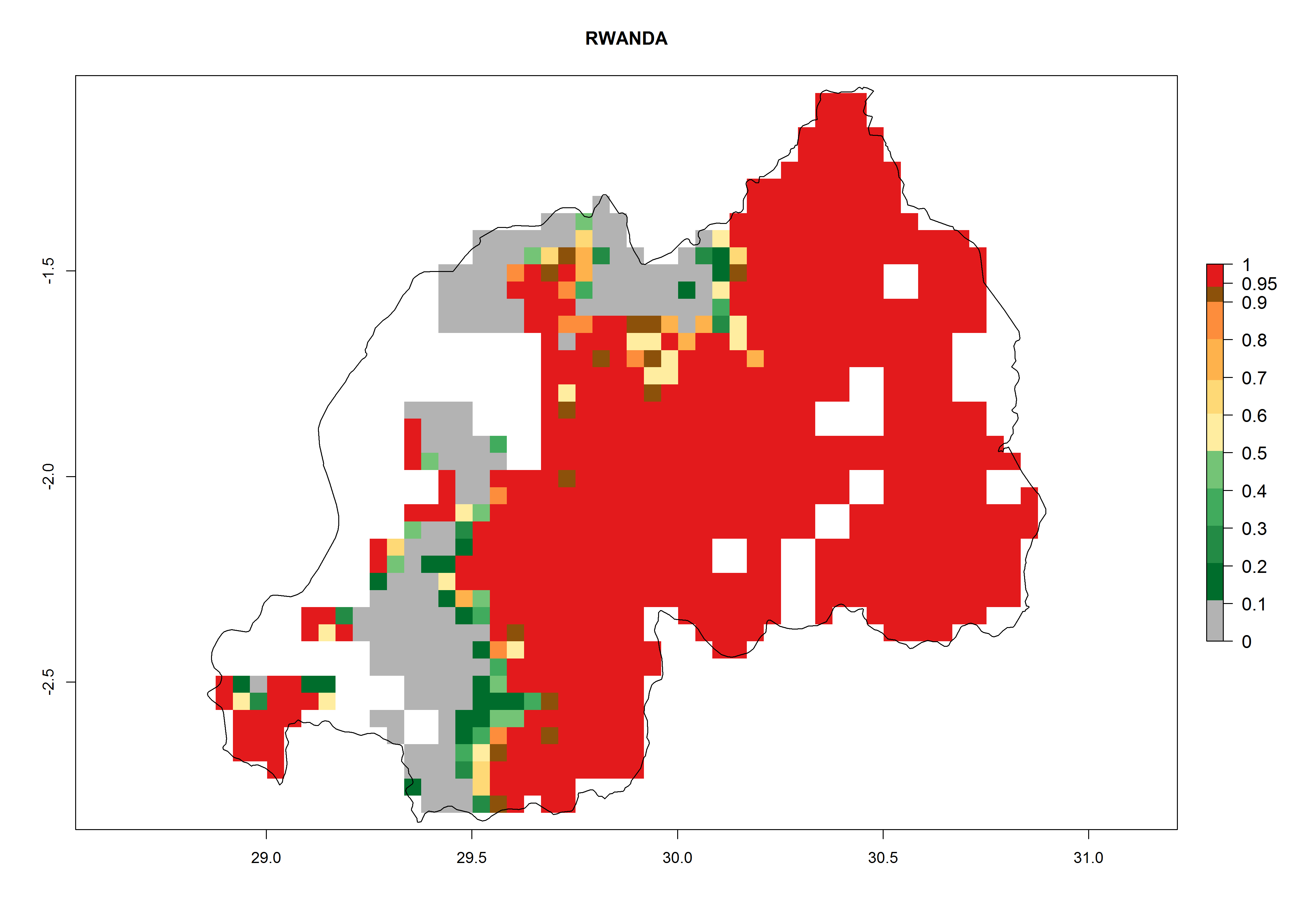
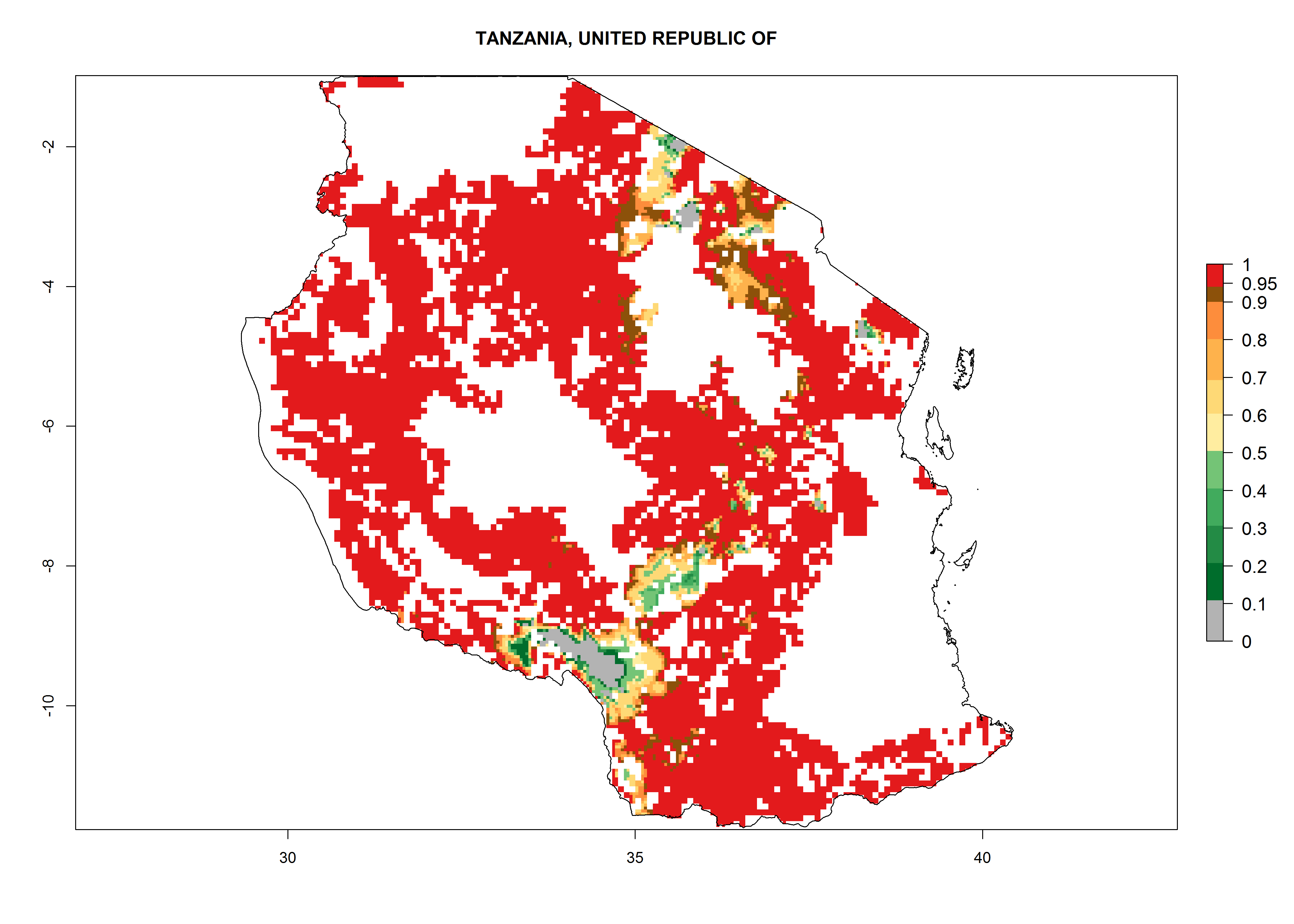
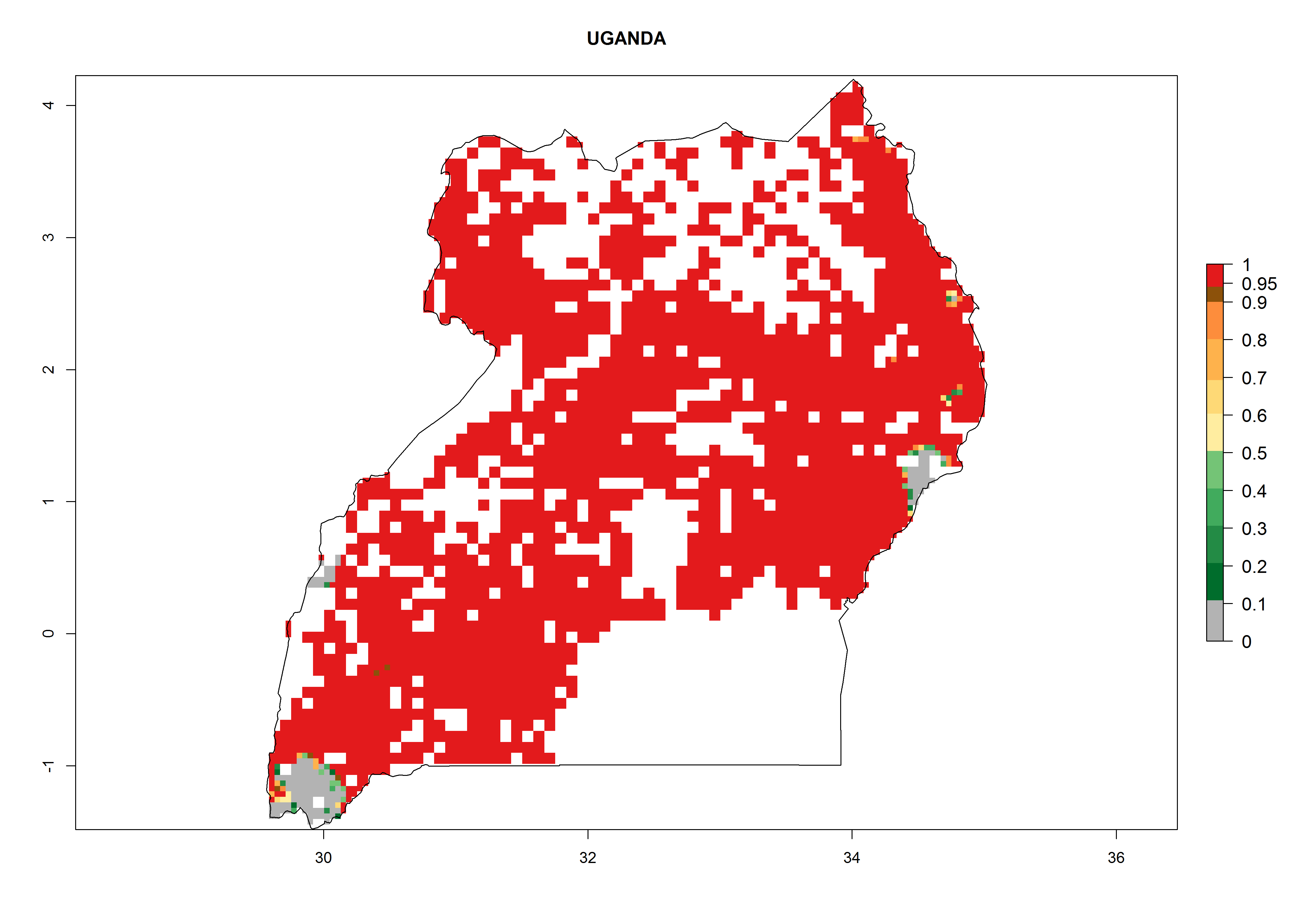
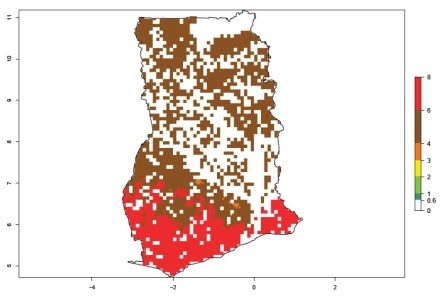
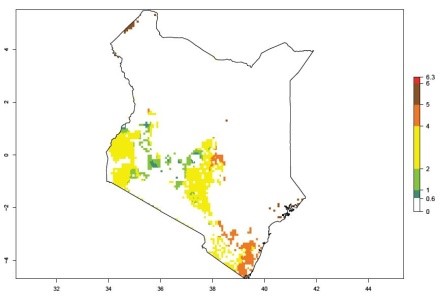
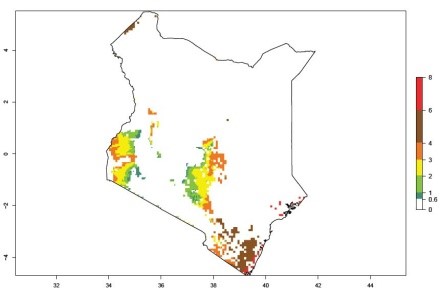
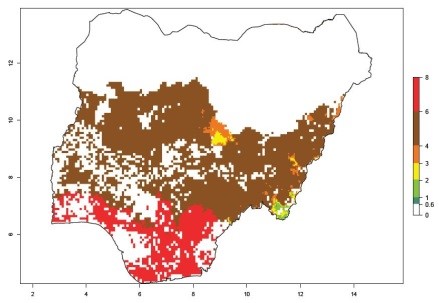
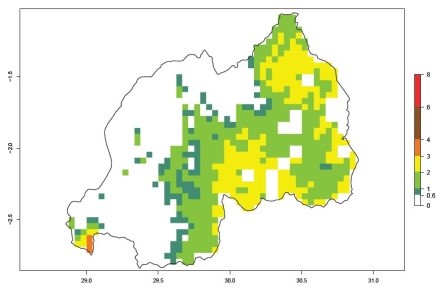
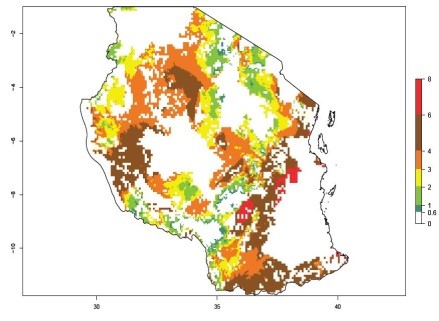
An ERI>0.95 in Uganda, Tanzania, Rwanda, Ghana, Nigeria, and Kenya reflects well the current distribution of C. brunneus under the current climate of the year 2000 (Fig. 6). Under the current temperatures, 4–6 generations per year can develop in most of Ghana and Nigeria (Fig. 6a, c); 1–5 generations per year in Kenya, Uganda, and Tanzania (Fig. 6b, f, e); and fewer (mostly 1–4) generations per year can develop in Rwanda (Fig. 6d). An AI up to 8 indicates a high population increase potential for Ghana and Nigeria (Fig. 6a, c). For Kenya, Uganda, and Tanzania, an AI up to 6 is estimated (Fig. 6b, f, e), whereas for Rwanda the population increase does not exceed an AI of 3 (Fig. 6d).
| ERI | GI | AI |
| a) Ghana |  |
 |
| b) Kenya
|
 |
 |
|
c) Nigeria |
 |
 |
| d) Rwanda | 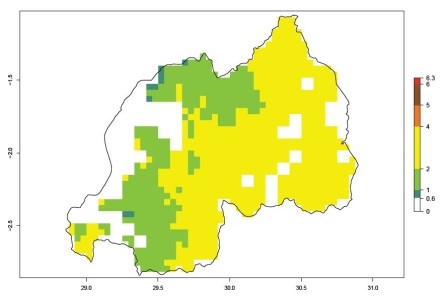 |
 |
| e) Tanzania | 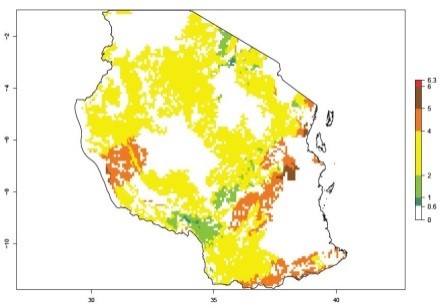 |
 |
| f) Uganda | 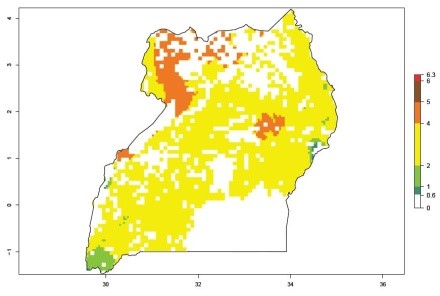 |
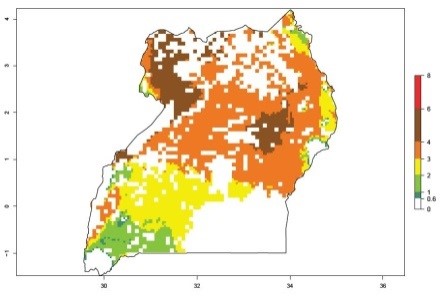 |
Figure 6. ERI, abundance (GI, potential damage), and activity (AI, potential population growth) of the sweetpotato weevil, Cylas brunneus, in sweetpotato production regions of selected African countries according to model predictions for the year 2000. An ERI>0.95 is associated with potential permanent establishment.
Phytosanitary measures
All C. brunneus developmental stages are within either the sweetpotato root tuber or stem. Therefore sweetpotato vines and roots from countries where the pest occurs must be inspected for presence of C. brunneus. Adult C. brunneus tend to lay eggs in older woodier parts of the vine; hence, tender sweetpotato vine tips (25–30 cm long) are recommended for use as planting material. A phytosanitary certificate should be provided for sweetpotato vines and for sweetpotato root tubers prior to export.
Adaptation to risk avoidance at farm level
The control strategies for C. brunneus are similar to those of C. puncticollis (see section 4.2.1).
Further reading
Alcazar, J., F. Cisneros, and A. Morales. 1997. Large-scale implementation of IPM for sweetpotato weevil in Cuba: a collaborative effort. Program Report 1995–1996. Lima, Peru: International Potato Center (CIP).
CABI. 2005b. Cylas brunneus (Fabricius). December (1st revision), Map No. 537. Distribution Maps of Plant pests. Wallingford, UK: CAB International. Available from http://www.cabi.org/dmpp/FullTextPDF/2006/20066600537.pdf.
European Plant Protection Organisation (EPPO). 2014b. Global database Cylas brunneus. European and Mediterranean Plant Protection Organization. Available from https://gd.eppo.int/taxon/ CYLABR.
Jansson, R.K., and K.V. Raman. 1991. Sweet potato pest management: a global perspective. International Potato Center, Lima, Peru.
Smit, N.E.J.M. 1997. Integrated pest management for sweetpotato in Eastern Africa. PhD thesis, Agricultural University Wageningen, Wageningen, Netherlands. 151pp.
Stathers, T.E., D. Rees, S. Kabi, L. Mbilinyi, N.E.J.M. Smit, H. Kiozya, S. Jeremiah, A. Nyango, and D. Jeffries. 2003. Sweetpotato infestation by Cylas spp. in East Africa: I: Cultivar differences in field infestation and the role of plant factors. International Journal of Pest Management 49: 131–140.