4.1.3 Potato Pests / Andean potato tuber moth, Symmetrischema tangolias (Gyen 1913)![]()
Synonyms: Trichotaphe tangolias (Gyen 1913)
Phthorimaea aquilina (Meyrick 1917)
Phthorimaea plaesiosema (Turner 1919)
(= Symmetrischema plaesiosema)
Phthorimaea melanoplintha (Meyrick 1926)
Gnorimoschema tuberosella (Busck 1931)
Taxonomic position: Insecta, Lepidoptera, Gelechiidae
Authors: M. Sporleder, P. Carhuapoma, & J. Kroschel
Common names
South American potato tuber moth, spotted potato tuber moth, tomato stemborer (in Australia) (English)
Hosts
Potato (Solanum tuberosum L.), tomato (Lycopersicon esculentum Mill.), sweet cucumber (Solanum muricatum Aiton), poroporo (Solanum aviculare G. Forst), kangaroo apple (Solanum laciniatum Aiton), black nightshade (Solanum nigrum L.), cayenne pepper (Capsium annuum L.)
Detection and identification
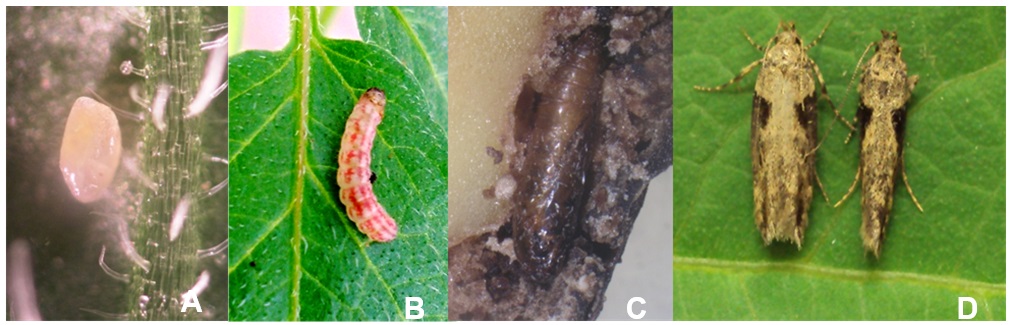
Larvae feed on tubers and stems of potato, which is the primary host plant of the Andean potato tuber moth. Neonate larvae penetrate into stems through plant axils, as evidenced by excrement deposited at the entry hole. The area around the entry hole becomes slightly discolored. Galleries made by larvae may run upward and downward within the stem (Photo 1A). Severe stem damage can cause the plant to wilt (tip death) or the infested stem to bend off (Photo 1B). Eggs can be found in slits of the stem. Pupation takes place amongst the debris of the host plant. Larvae enter into tubers through potato eyes. Initial infestation can be hard to detect at first, but after several days of mining larval excrements can be seen (Photo 1C). Larvae tunnel just under the surface or fully penetrate into the tuber. Severe infestation can cause entire tuber damage, leaving only debris (Photo 1D). Generally, larvae leave tubers before pupation through exit holes 2–3 mm in diameter. Infested tubers get a bitter taste and are unsuitable for human consumption or for livestock feed. Larvae mining activity and increased transpiration through the wounds cause tubers to lose weight and shrink. Wounds provide entry points for microorganisms that cause secondary infections.

Morphology
Egg
Freshly laid eggs are whitish oval (0.7 ´ 0.4 mm). During embryogenesis eggs become orange-yellow and turn dark gray shortly before the larvae hatch (Photo 2A).
Larva
The larva passes through five larval instars. Neonate larvae (L1) measure just 1 mm in length, and L5 reach 13 mm before pupation. Coloring of larvae depends on the feeding sources. Larvae mining in potato tubers appear whitish-beige, and those mining in plant stems are greenish—both with a dark head capsule. Characteristic for larvae are three reddish longitudinal stripes on the upper part of the thorax and abdomen, which appear at the L3 instar (Photo 2B).
Pupa
Pupae are 7–8 mm long, first brownish in color, later turning dark-brown and almost black 1 day before emergence (Photo 2C).
Adult
The adult moth is brownish-gray (silver-gray), with characteristic black triangular spots at the lateral edges of the forewings. Fine hairs fringe the edges of the forewings. The hindwings, which are shorter and narrower than the forewings, are bordered with pale-ocher scales. The resting moth is 9–12 mm long, with a body length of 6–9 mm. Wingspan is about 18–19 mm. Males are smaller than females, with a cone-shaped abdomen; the female abdomen is wider with a blunt ending (Photo 2D).
Biology
Adult females lay approximately 140–185 eggs either individually or in small clusters, mainly into leaf axils of their host plants or on potato tubers. Fully grown L5 instar larvae usually leave the feeding medium and spin a silken cocoon on plant epidermis or plant debris. Occasionally, pupation occurs inside tubers and plant stems, which can be hard to detect in the closely related common potato tuber moth [Phthorimaea operculella (Zeller)]. The species is multivoltine, producing overlapping generations (i.e., all life stages are found at the same time in potato fields or stores). No signs of hibernation have been reported.
Temperature-dependent development
The life cycle depends strongly on prevailing temperature. According to the model established, development is possible within the temperature range of <10ºC to approximately 28ºC (see Annex 7.3.3). At 10°C, the median immature development time is about 196 days; however, with rising temperatures the development time decreases and is about 36 days only at the pest’s upper temperature limit of 28°C. The lower temperature threshold for survival in larvae is around 8°C (only about 6% of the newborn survive to the adult stage). Survival rates might be higher, even at lower temperatures, if the larvae are exposed to these temperatures intermittently. Survival in eggs and pupae is generally >80%, even though survival declines in both stages at extreme temperatures <8°C and >25°C. The lifespan of adults decreases with rising temperatures, from about 36 days at 10°C to about 8 days at 28°C. Females live about 4% longer than males. Oviposition peaks at temperatures around 17°C, with about 148 (±30) eggs per female; 50% of the eggs are laid at this temperature within 10 days. Reproduction declines as temperature deviates from this optimum temperature, and the median oviposition time declines as temperatures rise and extends as temperatures decrease. At 8°C reproduction per female reduces to 35 (±7) eggs, whereas 50% of the eggs are laid within 14 days. At 28°C only 22 (±5) eggs are produced per female, and the median oviposition time shrinks to 6 days.
The models established to describe the development times, survivorship, and reproduction in the species (see Annex 7.3.3) were assembled into an overall phenology model that allows the specie’s life-table parameters to be estimated according to temperature. The predicted intrinsic rate of increase (rm) indicates that populations may establish and grow within the temperature range of 8°–28°C; the highest population growth can be expected at 21°C. At this optimum temperature, population size increases potentially 5.3% per day (i.e., populations double within 13.3 days). These simulations indicate that S. tangolias is much more adapted to cooler and less adapted to warmer temperatures than P. operculella; S. tangolias performs better at temperatures <15°C and has a lower temperature threshold than P. operculella. In the Andean region, S. tangolias develops 3–5 generations per year. In most mid-elevated zones of the Andes, the whole cycle from egg to egg lasts over 100 days.
Means of movement and dispersal
Dispersal of S. tangolias is similar to that of P. operculella. Adults disperse in short “hopping” flights near the ground with the aid of prevailing winds. All immature stages are moved with infested tubers over short and long distances. Infested seed can initiate field infestations and infested tubers at harvest initiate infestations in potato stores. Infested potato tubers have likely facilitated the dispersal over long distances around the globe, although S. tangolias much less successfully than P. operculella.
Economic impact
S. tangolias is a serious pest of potato in its region of origin, the Andes of South America. It has become an economically significant pest under potato field and storage conditions in mid-elevated regions of the Andes (Peru, Bolivia, Ecuador); its status in Colombia is not well confirmed. It is not a pest at low altitudes but has displaced P. operculella in many mid-elevated valleys. However, both species coexist in zones where temperature conditions are within the thermal limits of each species. Coexistence of both species can result in higher crop damage than the additive damage expected from each species alone. This increasing crop damage is due to better resource utilization (feeding complementarities). In the Andes, losses in the field may reach up to 30%, but most economically significant damage occurs when infested tubers are transferred to potato stores where re-infestation takes place. Without adequate protection, farmers can completely lose their house-stored potatoes within 3–4 months of storage time. In Australia and New Zealand, where the pest was accidentally introduced from South America, this Microlepidoptera is more recognized as a local pest of tomato, poroporo, sweet cucumber, and other solanaceous crops and commonly referred to as “tomato stemborer.” At national level, however, it is considered a minor pest and its economic impact on these crops is not well reported in the literature.
Geographical distribution
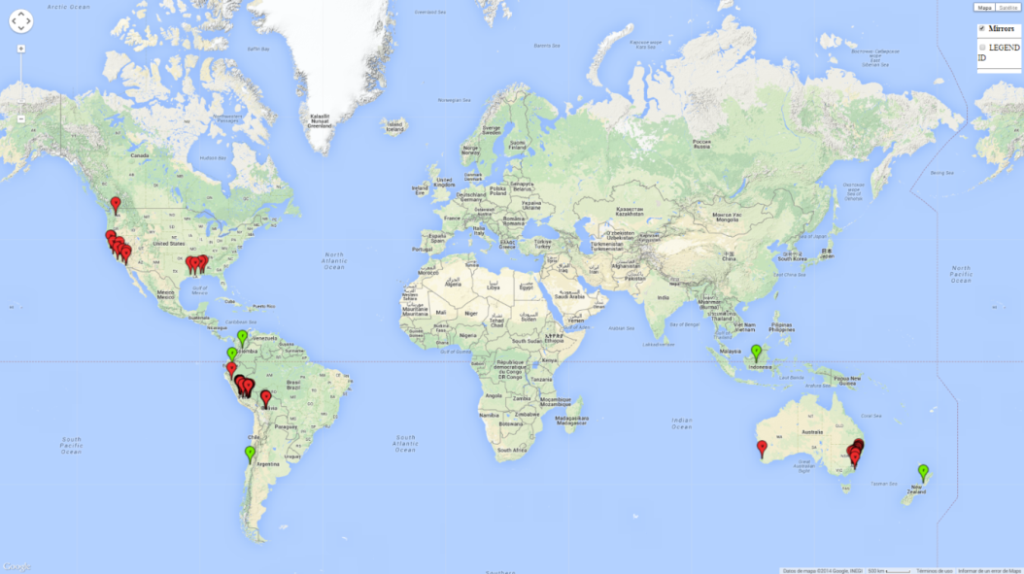
Native to South America (Peru and Bolivia) the pest has spread to other regions during the last several decades (Fig. 1). Records include North America, Australia, New Zealand, and, more recently, Indonesia and Chile. In South America and the Australian region, this species is known as a pest of potato and tomato; however, in North America it does not attack these crops but feeds on black nightshade.
| Asia | Indonesia |
| North America | USA (California, Washington State, Mississippi) |
| South America | Peru, Bolivia, Colombia, Ecuador, Chile |
| Oceania | Australia, New Zealand |
Phytosanitary risks
S. tangolias is currently present in potato-growing zones in the northern Andean region of South America, and is recorded in North America, Australia, and New Zealand. Recently it was recorded in Indonesia and Chile. Therefore, the pest can be considered a global one, which makes prevention of its further distribution necessary (particularly in the South American and Asian regions). Several countries in the Americas and Asia have included S. tangolias in the list of quarantine pests (e.g., Mexico, Costa Rica, Thailand, Vietnam, and others). Potato-producing countries in Africa, particularly those where the risk maps presented below reveal establishment risk, should also consider S. tangolias as a potential threat to national potato production. As potato is the prime host crop, introduction of S. tangolias will be strongly associated with the pathway of importing and the formal and informal movement of seed tubers. Therefore, S. tangolias requires phytosanitary measures in countries that import potato tubers from major source countries (e.g., Ethiopia, which imports potato tubers from Peru). S. tangolias also attacks tomato, although tomatoes are grown in environments that are generally warmer than the temperatures that favor the growth of S. tangolias populations.
Risks mapping under current and future climates
Global Risks
Changes in establishment and future distribution
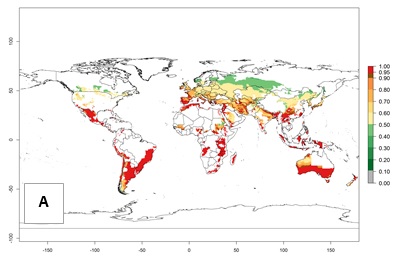
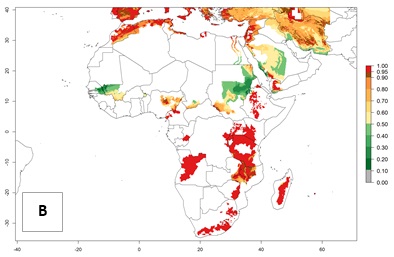
Figure 2 illustrates the establishment risk index (ERI) predicted for the current climate scenario (year 2000) and for a climate change scenario (year 2050) on a worldwide scale. For the Andean region, an ERI>0.95–1 corresponds well with high-elevated potato production areas (>2,800 masl) where S. tangolias prevails today, particularly in the northern Andes, including northern Peru, Ecuador, Colombia, and Venezuela. However, S. tangolias also appears quite frequently in zones where the predicted ERI-value decreases to 0.95. Such areas include the assumed origin of the pest (i.e., the high-elevated Andean region in southern Peru and Bolivia) and some locations where the pest was recorded recently as an established non-native species—that is, the coastal region of Chile, elevated regions in Indonesia, Australia (New South Wales, Western Australia), and the United States (California).
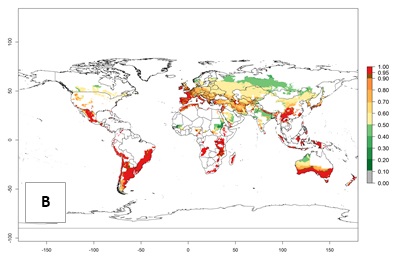
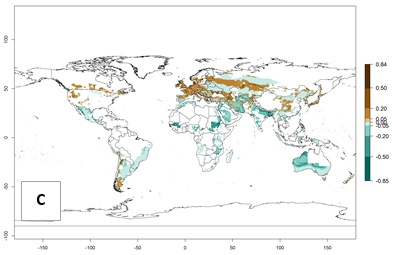
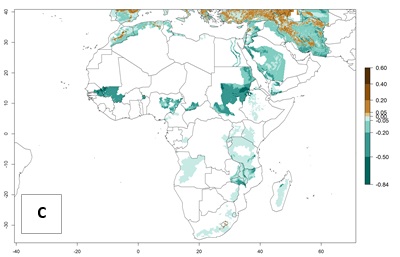
Figure 2. Changes in establishment and potential distribution of the Andean potato tuber moth, S. tangolias, in potato production areas worldwide according to model predictions, using the ERI for the years 2000 (A) and 2050 (B), and changes of the ERI between 2000 and 2050 (C). An ERI>0.95 is associated with potential permanent establishment.
These predictions show that S. tangolias potentially could establish in elevated potato production zones in Central America (Costa Rica, Guatemala, Mexico), North America (southern United States, Florida), and the Caribbean (Dominican Republic, Haiti, Cuba). In the Asia-Pacific region, predictions show that the pest could establish in all mountainous regions of Indonesia, Malaysia, Papua New Guinea, Philippines, Vietnam, southern India, and Sri Lanka, as well as in the Hindu Kush-Himalayan region (India, Nepal, Bangladesh, Bhutan, Myanmar, China). In Africa, the pest could potentially establish in almost all potato production zones, except in areas in the Sahel region (Sudan, Burkina Faso, Mali, Mauretania, Senegal). The potato production area in the Sahel, however, is about 7,000 ha less than 0.5% of the total production area in Africa. The simulation shows that the pest could also establish in Europe and the Middle East. The ERI is >0.95 in southern Europe (Mediterranean countries), including Portugal, Spain, southern Italy (e.g., Sicilia and Sardinia); southern France, Greece, and Cyprus; and in the Middle East, including Turkey, Syria, Israel, Jordan, Saudi Arabia, and Yemen.
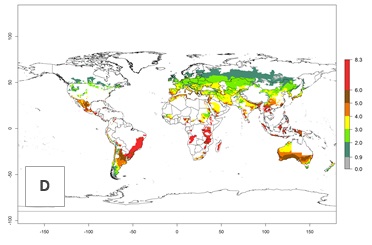
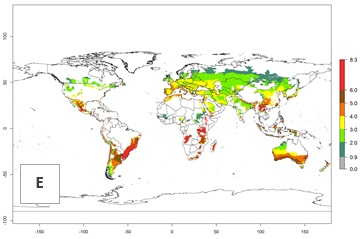
With the current predictions on rising temperatures due to climate change, it seems that the establishment potential for S. tangolias decreases in most subtropical and tropical regions but increases in temperate regions (see Fig. 2B). S. tangolias may become established in some additional countries, particularly in Europe, where it might extend its establishment range northward, particularly in Spain, France, and Italy, and possibly Ireland. On the other hand, the establishment risk decreases in the Mediterranean region (southern Spain, southern Italy, Greece, Cyprus, Turkey). In the Middle East, the establishment risk is expected to decrease, with the exception of Turkey and Yemen. In Africa, the establishment risk decreases in all regions but remains high (ERI>0.95) in Southern Africa (Southern Hemisphere) and in the western Mediterranean region (Morocco, Algeria, Tunisia). In the Andean region, the pest might shift into higher altitudes. The establishment risk is still high in the mountainous potato production areas in Asia and Africa as mentioned above. But the pest is expected to shift into higher altitudes with a decrease in overall area under risk.
Changes in abundance
In the high-elevated Andean region, where S. tangolias prevails today, the pest develops 2–4 generations per year. In these locations the estimated activity risk (AI) is 1–3, which denotes that S. tangolias populations grow between 0.6% (AI=1) and 1.9% (AI=3) per day averaged over the year. In regions that show high risks for S. tangolias establishment in Asia and Africa (Fig. 3), the predicted numbers of generations per year are higher than for the Andean region. In Central Africa, 4–6 generations per year are expected—for example, in Rwanda—whereas in lower areas (e.g., Uganda) the expected number of generations per year is 6–8. Accordingly, the activity or spread potential is much higher in Asian and African potato-producing regions than in the Andes.
Global warming is likely to increase the potential numbers of generations per year, due to faster development in immature life stages. The activity is expected to reduce, however, in almost all tropical and subtropical potato production zones worldwide under climate change scenarios (Fig. 3F) due to reduced survivorship and reproduction. Increased activity is expected in tropical and subtropical mountains—the pest might shift into higher altitudes—and in temperate regions the pest shifts northwards and southwards in the Northern and Southern hemispheres, respectively.
| GI | AI | |
| 2000 | 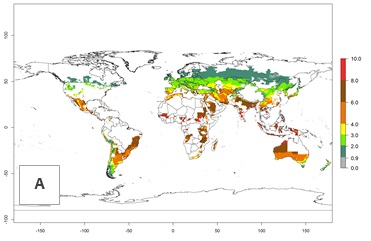 |
 |
| 2050 | 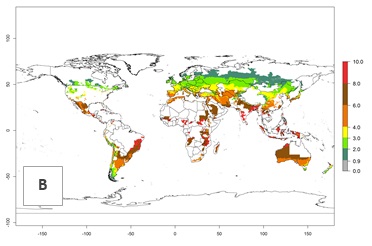 |
 |
| Index change (2000 – 2050) | 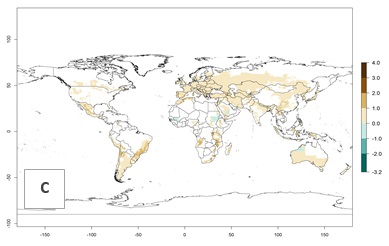 |
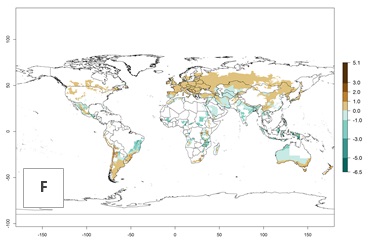 |
Figure 3. Changes in abundance (generation index [GI], damage potential) and activity (AI, potential population growth) of the Andean potato tuber moth, S. tangolias, in potato production regions worldwide according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
Regional Risks for Africa
Changes in establishment and future distribution
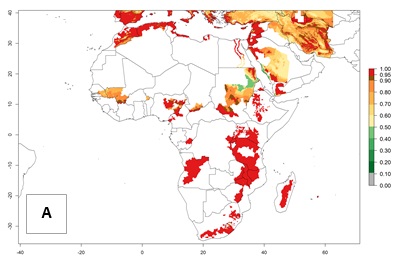
High risks for establishment are in major potato production zones in Central and Southern African highlands (Fig. 4).
Figure 4. Changes in establishment and potential distribution of the Andean potato tuber moth, S. tangolias, in potato production regions in Africa according to model predictions, using the ERI for the years 2000 (A) and 2050 (B), and changes of the ERI between 2000 and 2050 (C). An ERI>0.95 is associated with potential permanent establishment.
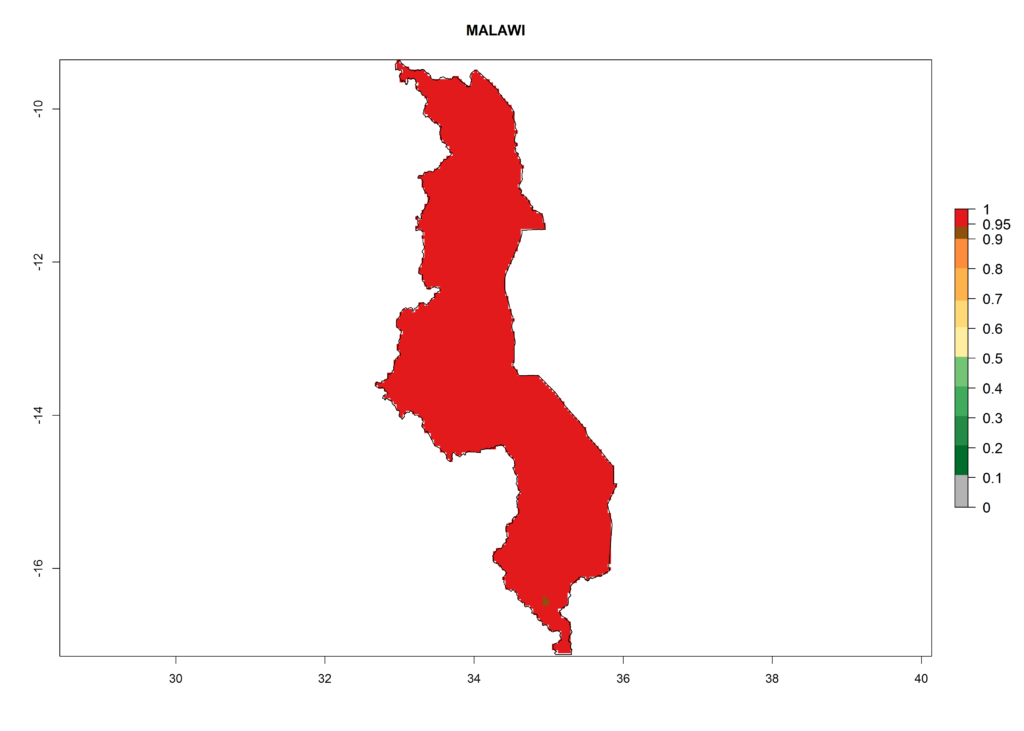
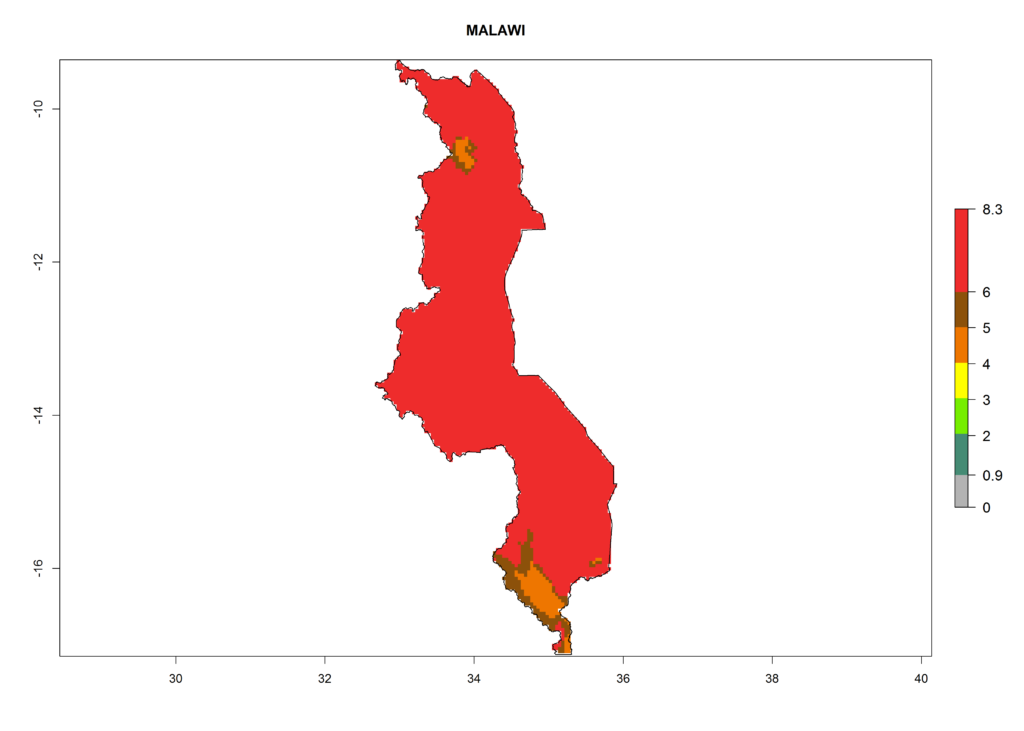
It seems that global warming will generally decrease the establishment risk in African countries. The likelihood of establishment can be expected to significantly decrease in the Sahel region and its tropical savannas; however, it is not clear whether potato production remains possible in these areas under a climate change scenario. Although the establishment risk is expected to decrease in all African countries, the establishment risk remains high (>0.95) in the Mediterranean south coast and Southern Africa (south of the Sahel and Horn of Africa). Only in the mountainous region of Zimbabwe, Mozambique, and Malawi might the establishment risk become significantly limited in certain potato production areas as a result of global warming.
Changes in abundance
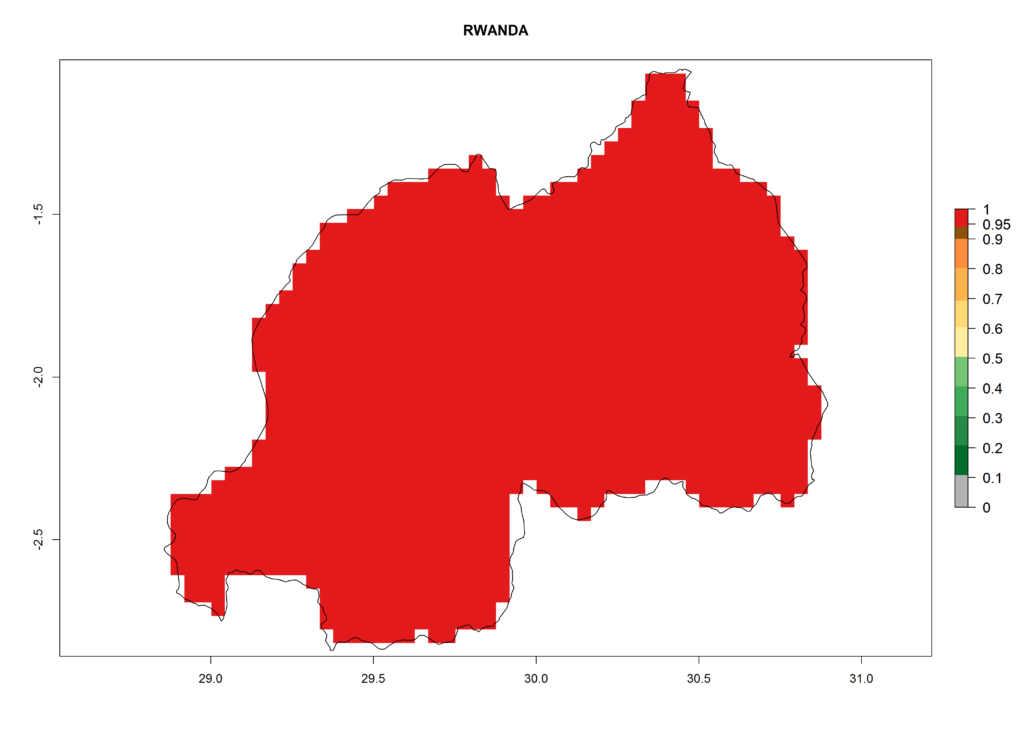
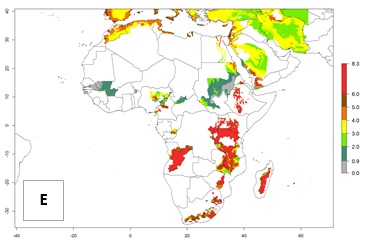
In high-elevated regions, up to 6 generations per year could develop; in lower altitudes 6–8 generations per year are possible (Fig. 5). The activity (i.e., population increase and spread and damage potential) is expected to be high in Central Africa, where it almost reaches the maximum population growth rate of the species (i.e., of about 5% population increases per day). Therefore, the species represents a potential threat to potato production especially in highly populated countries such as Rwanda and Burundi, where potato represents an important crop for food security. Such countries should consider the species as a potential threat to agricultural production and food security.
| GI | AI | |
| 2000 | 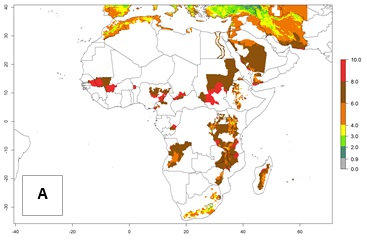 |
 |
| 2050 | 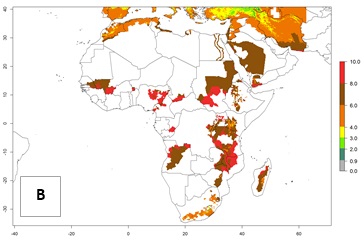 |
 |
| Index change (2000 – 2050) | 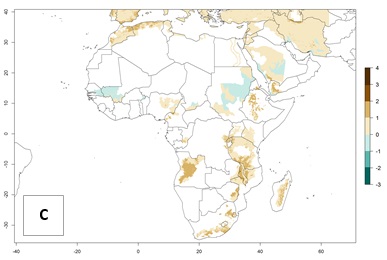 |
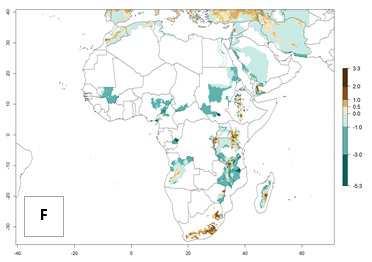 |
Figure 5. Changes in abundance (GI, damage potential) and activity (AI, potential population growth) of the Andean potato tuber moth, S. tangolias, in potato production regions of Africa according to model predictions, using the GI (A, B) and AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
Country Risk Maps
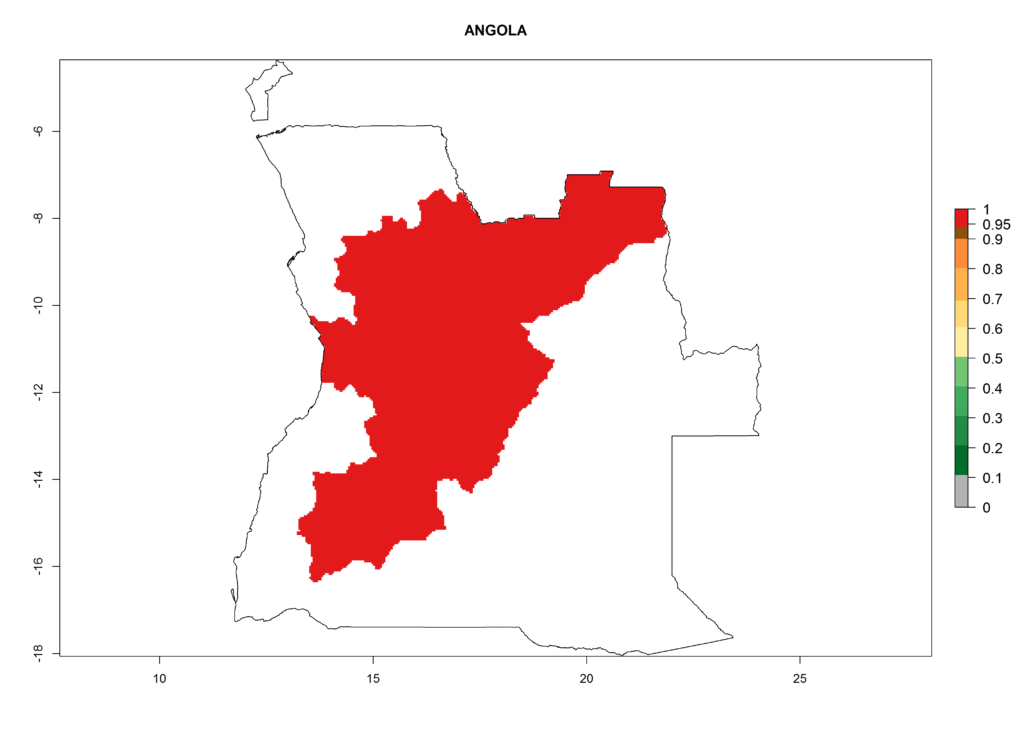
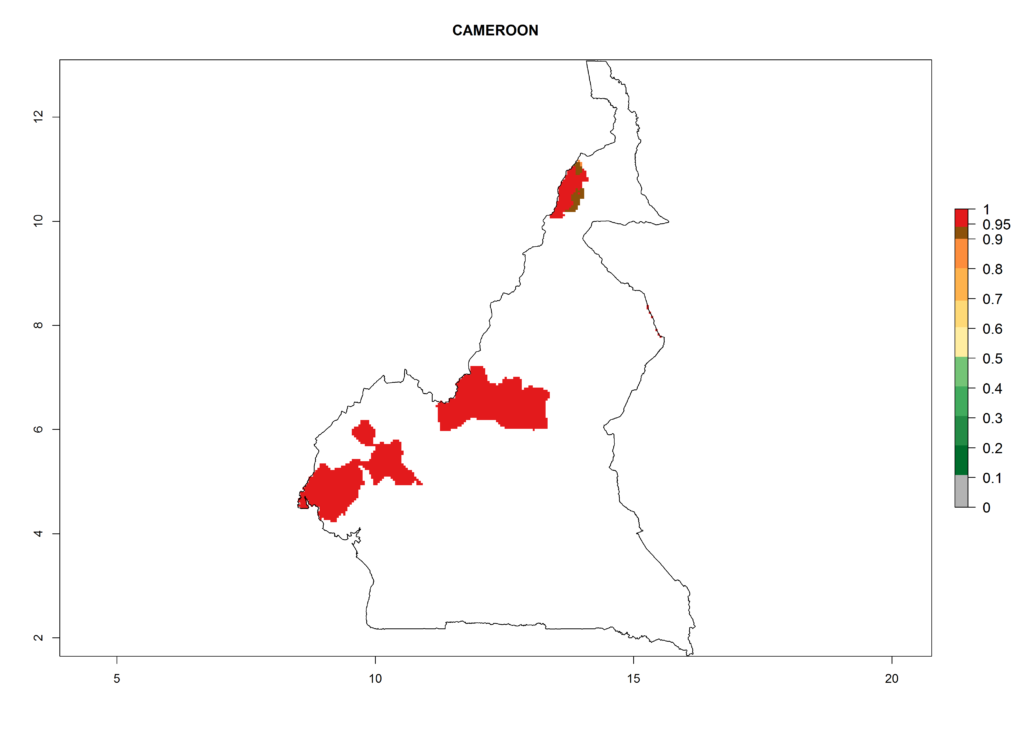
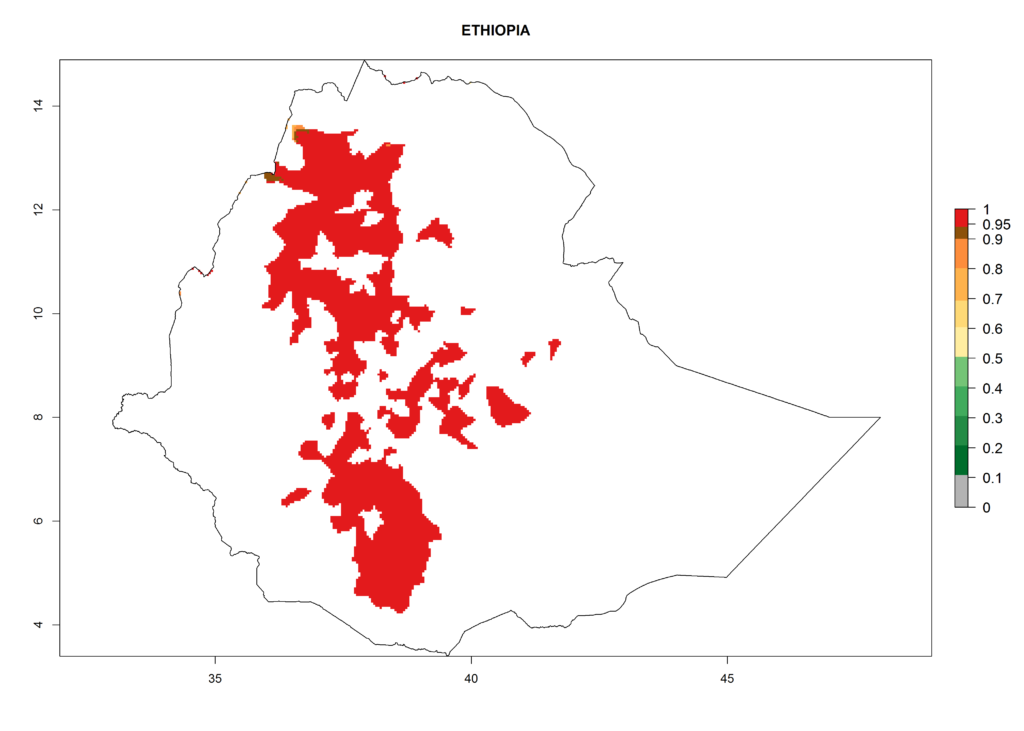
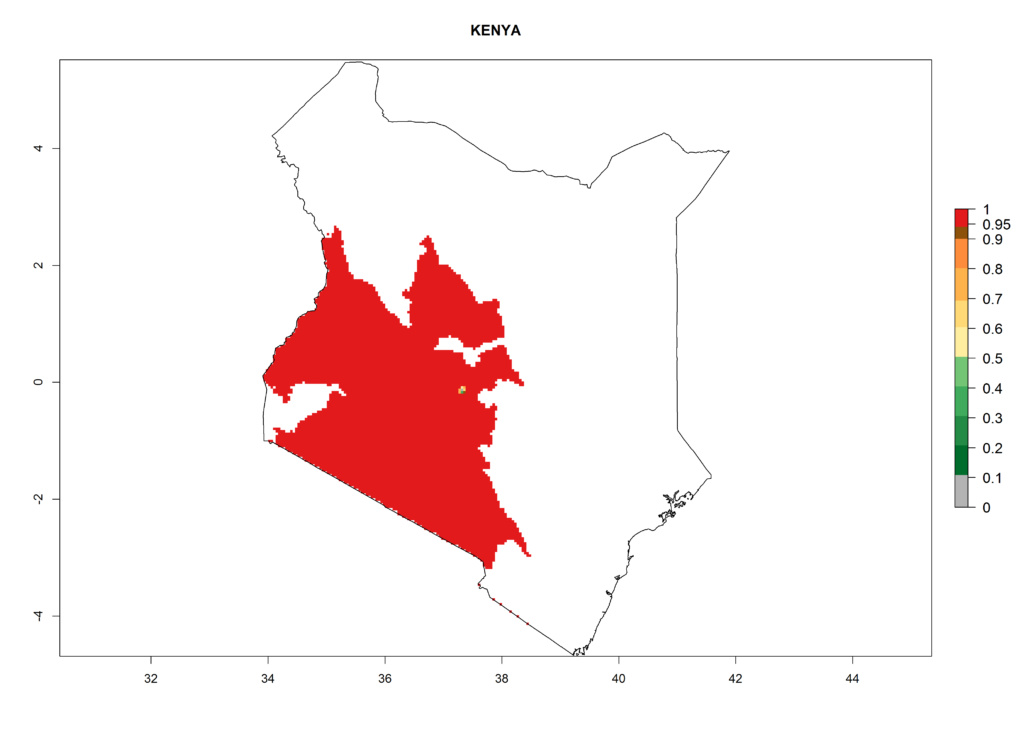
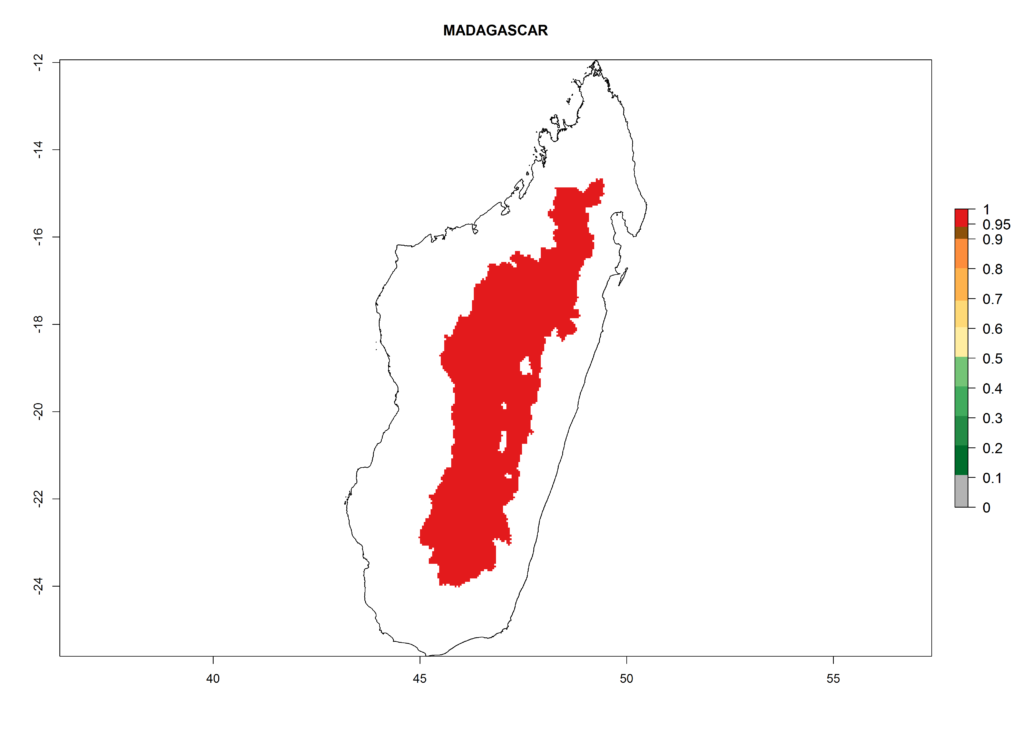
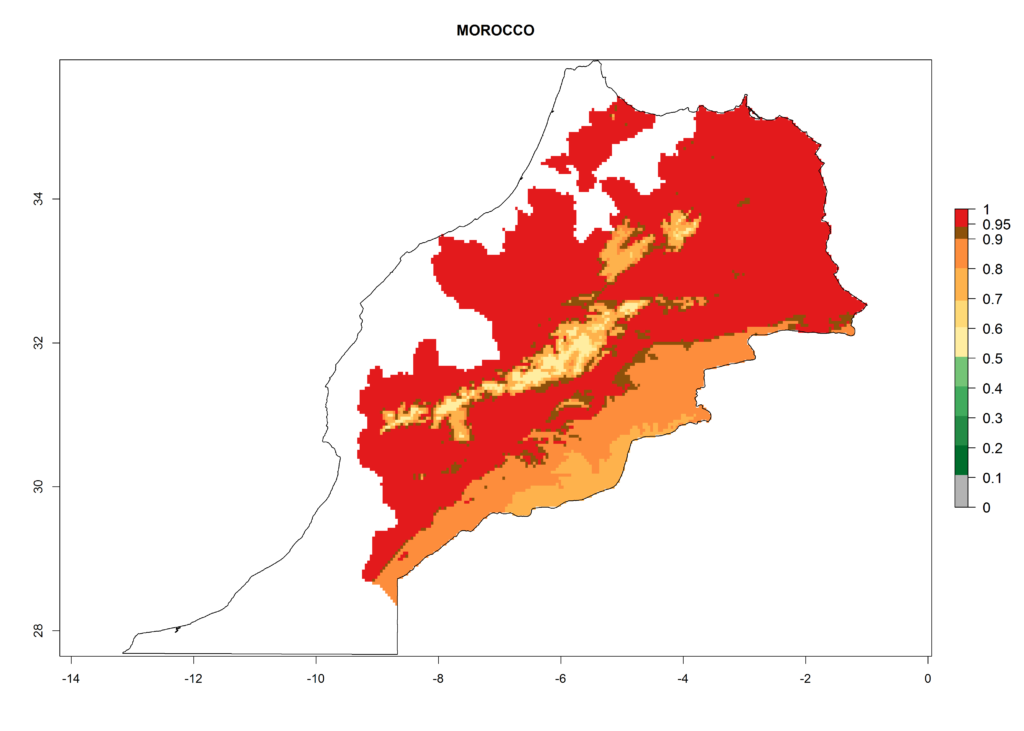
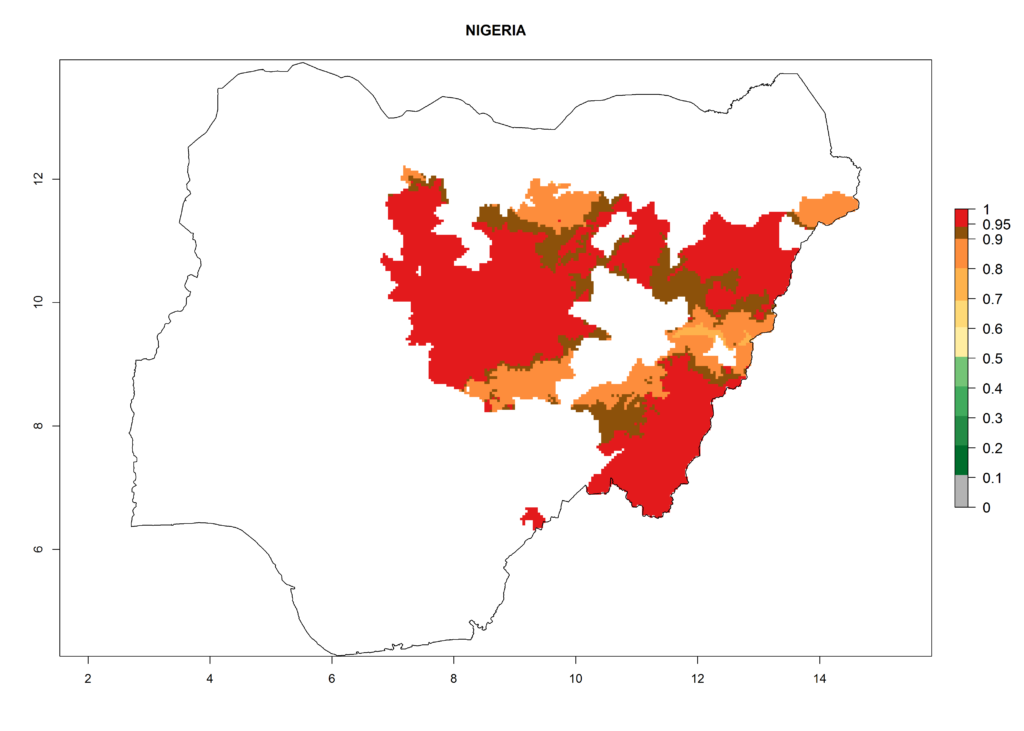
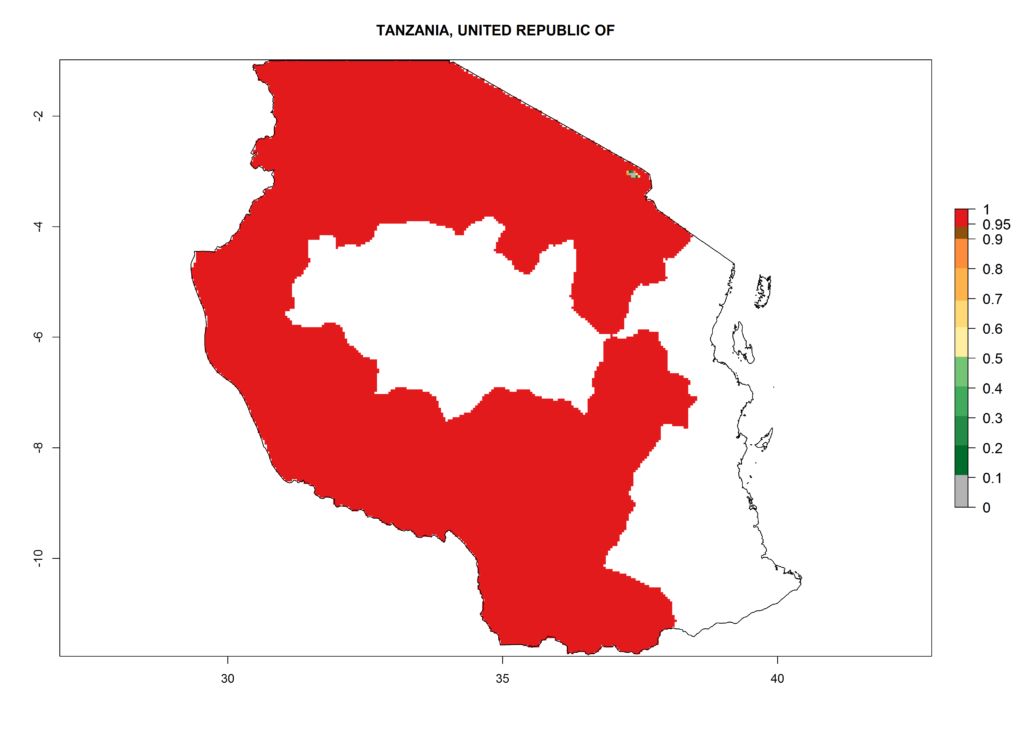
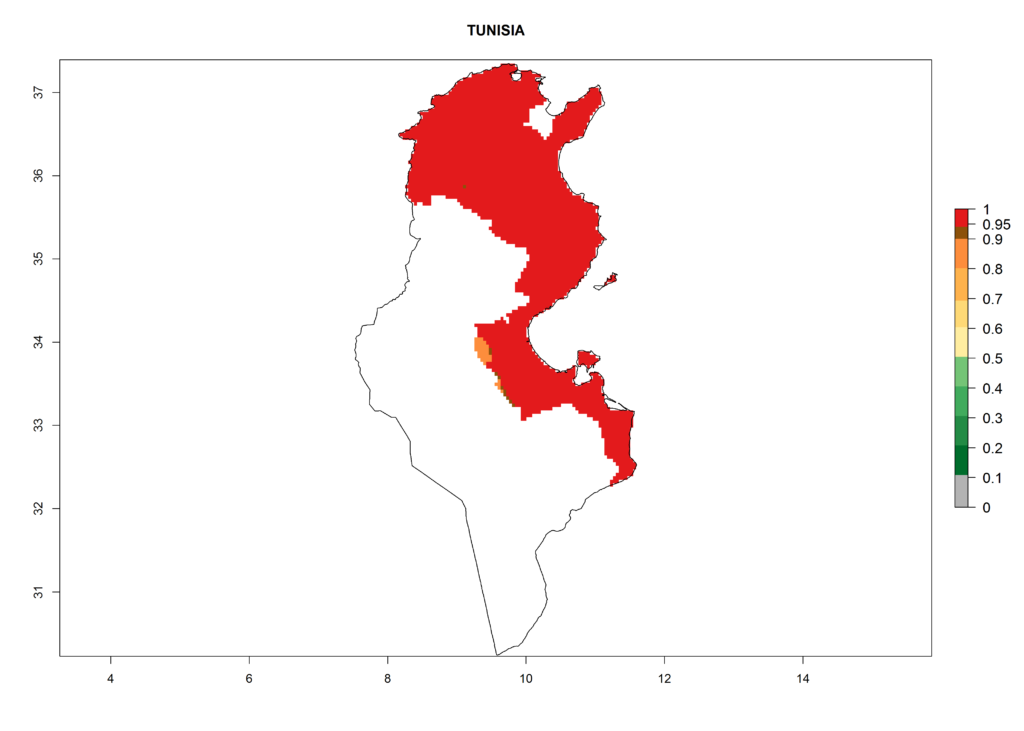
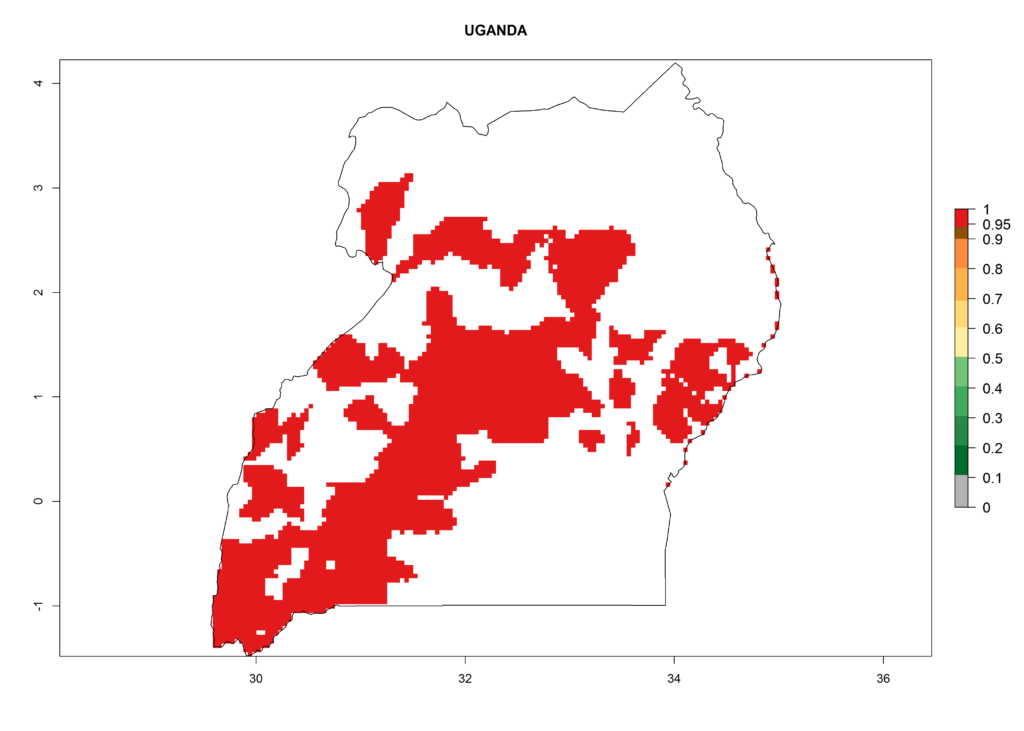
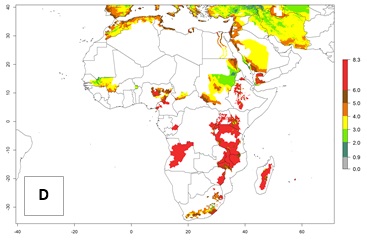
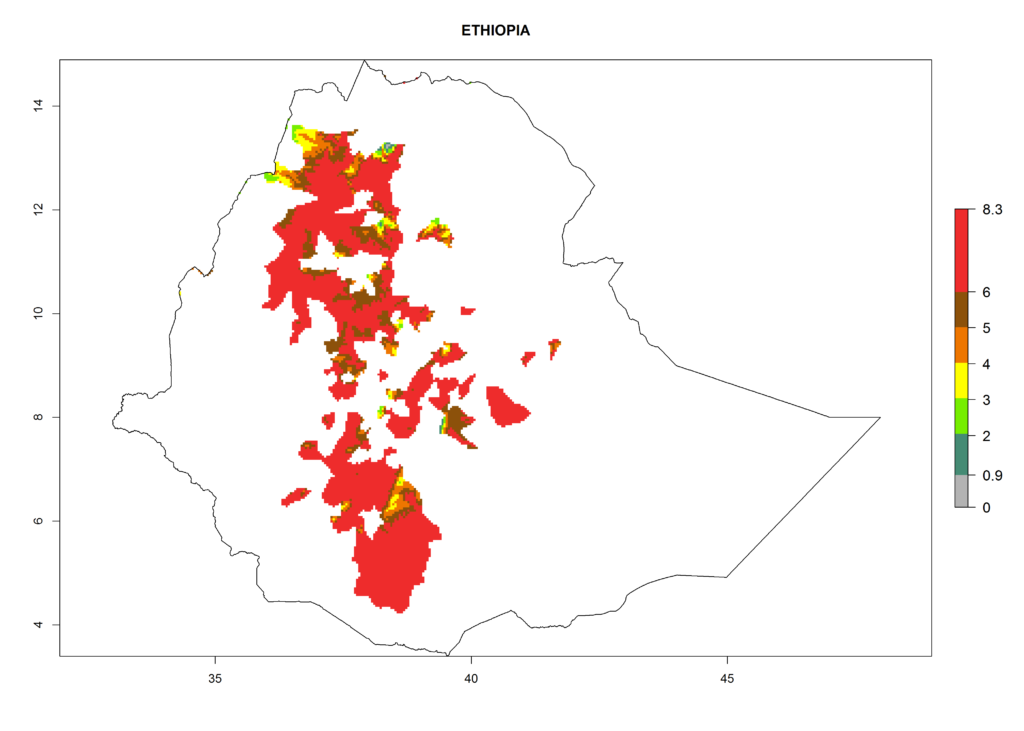
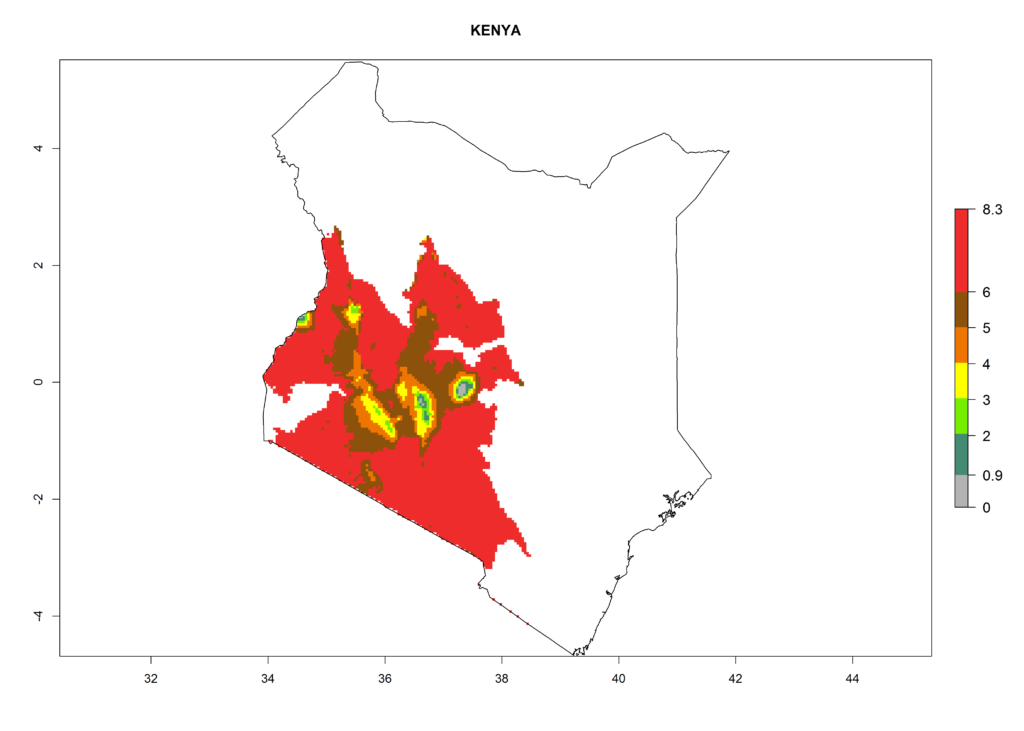
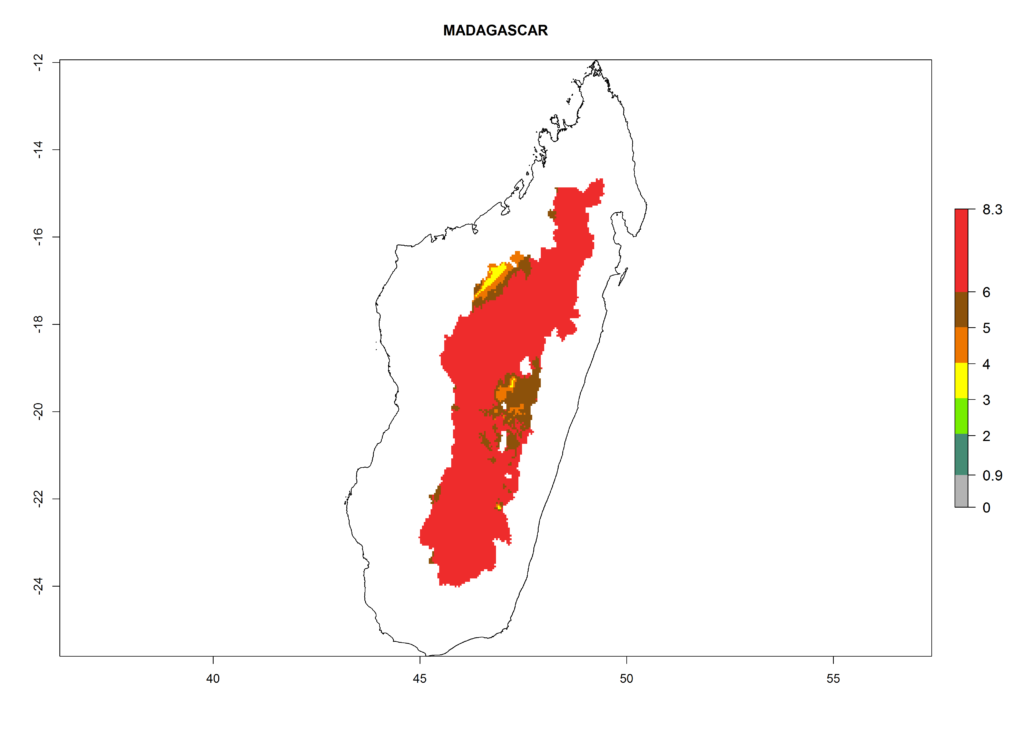
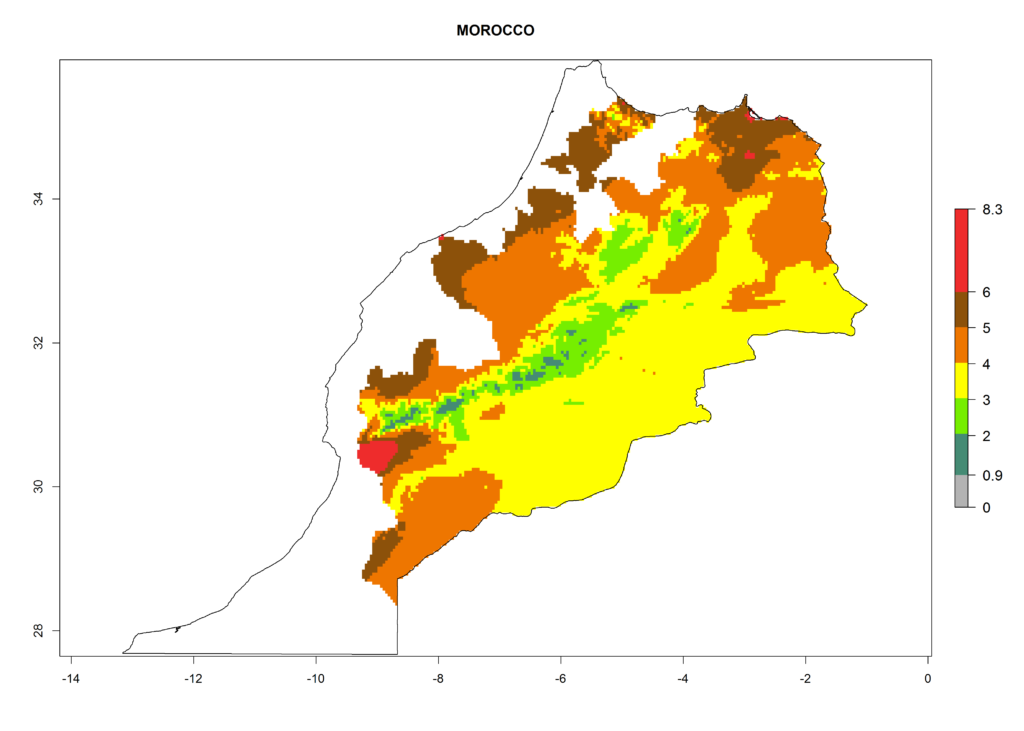
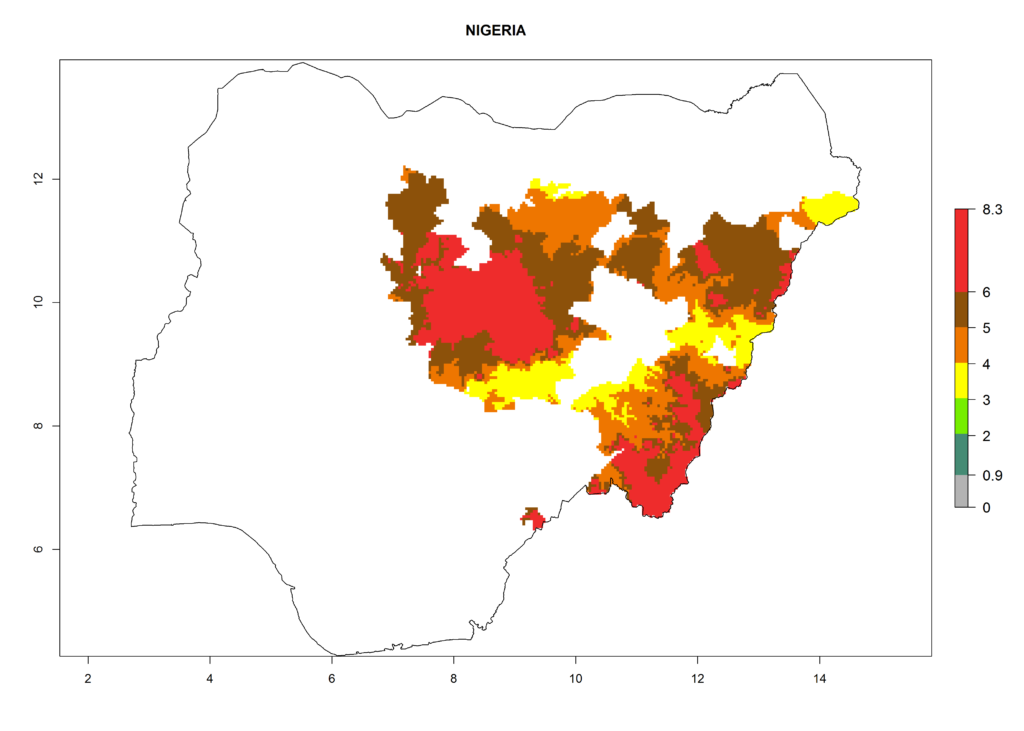
In North Africa, the establishment of S. tangolias is potentially possible in potato production regions of Morocco and northern Tunisia (Fig. 6). The risk of S. tangolias is considered quite high in the mountainous potato production regions of Kenya, Rwanda, Tanzania, and Uganda (Fig. 6), which cover about 32% of the total African potato production area. In these countries the establishment risk and spread potential are high (ERI>0.95-1, AI>6). Further, an ERI>0.95 is projected for almost all potato areas of Angola, Cameroon, Ethiopia, Madagascar, Malawi, and Nigeria.
| ERI | GI | AI |
| a) Angola | 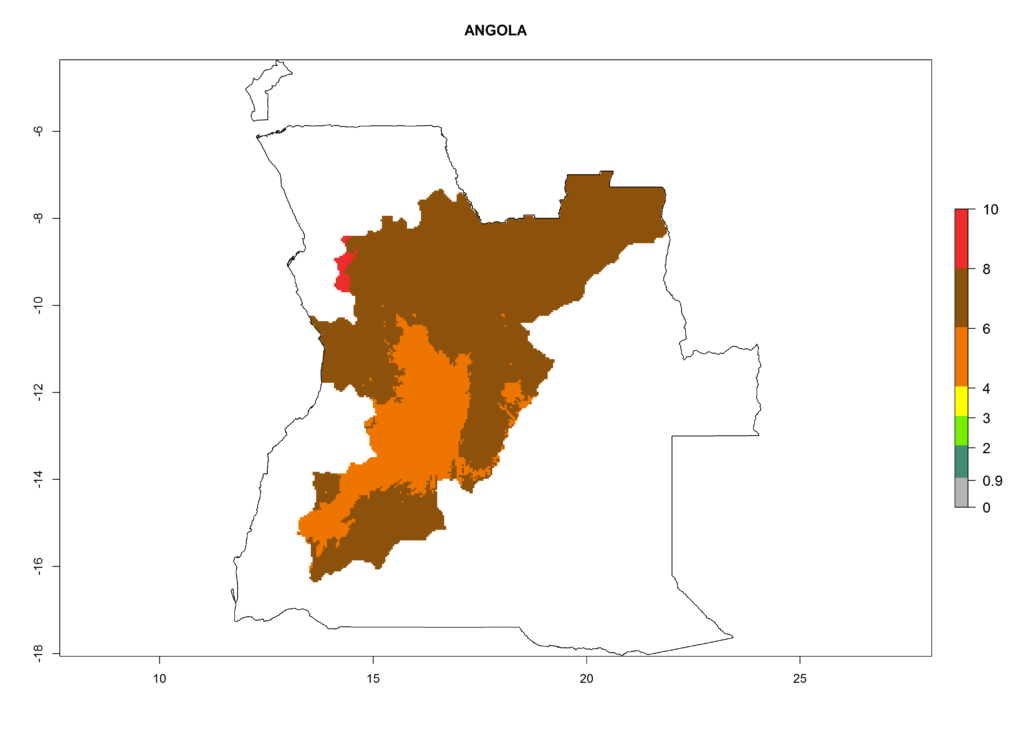 |
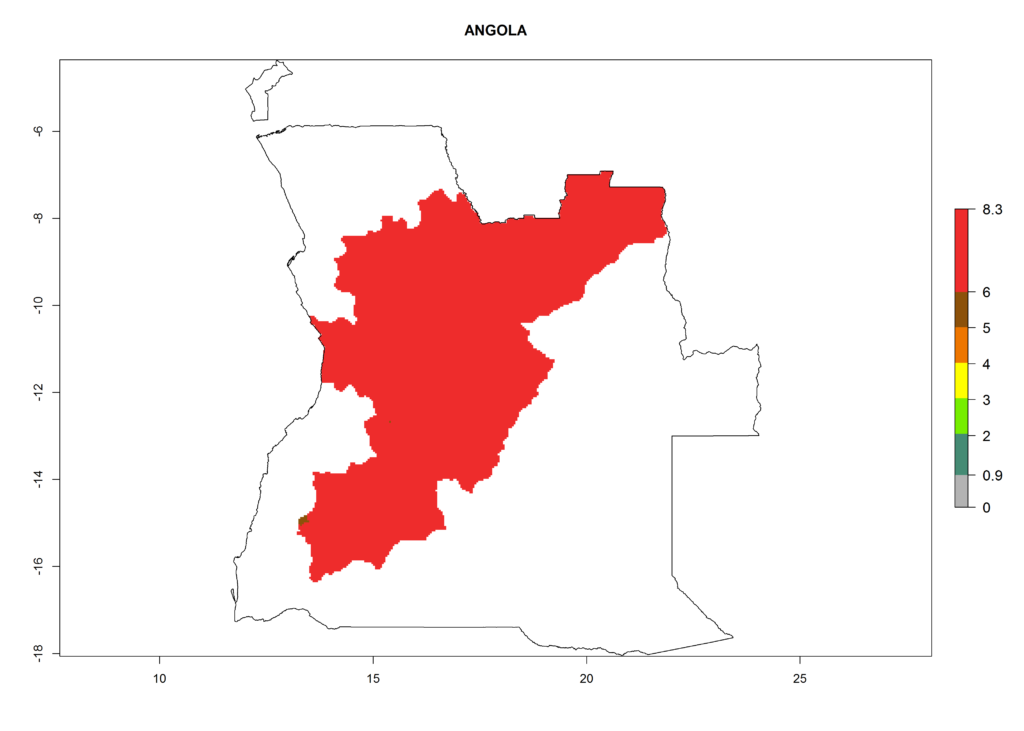 |
| b) Cameroon | 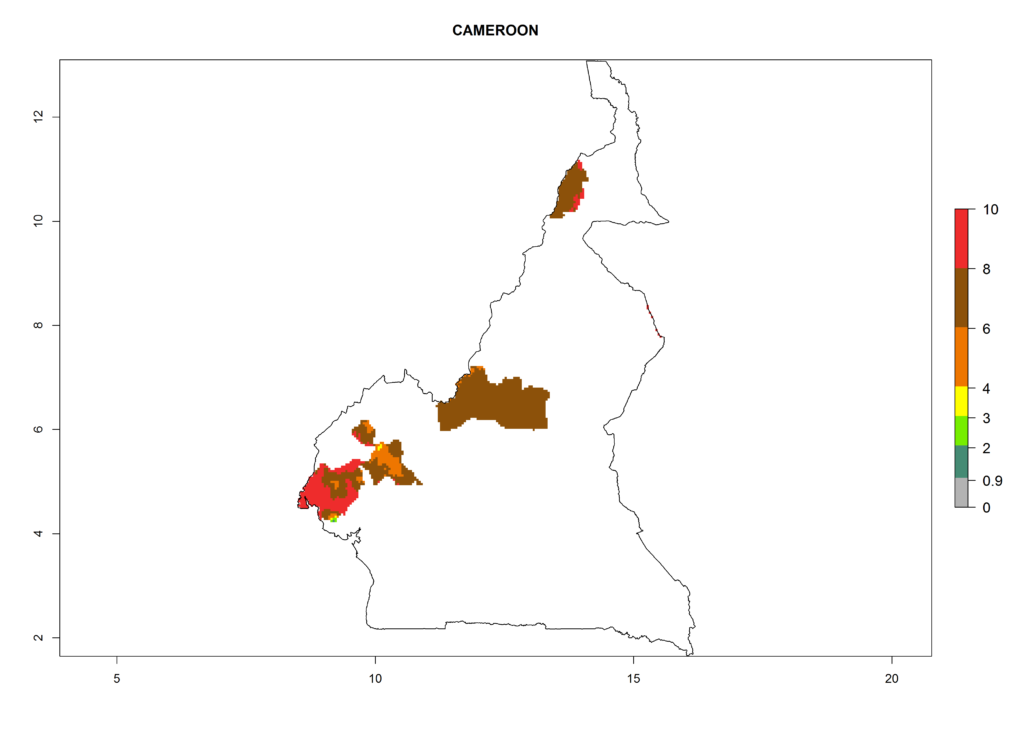 |
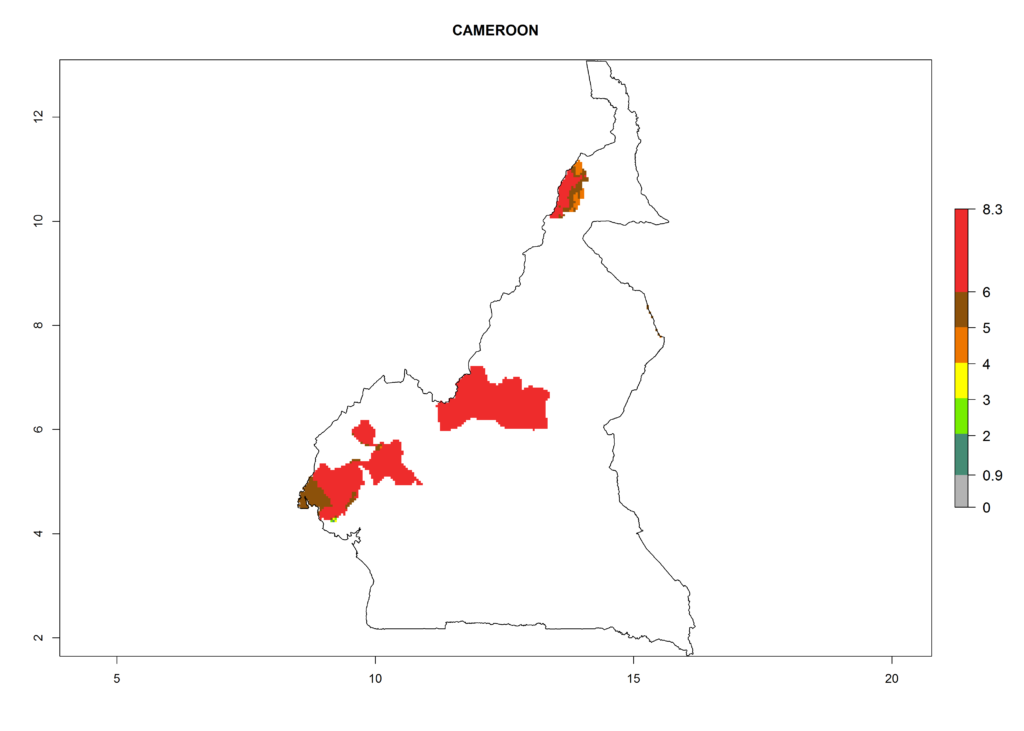 |
| c) Ethiopia | 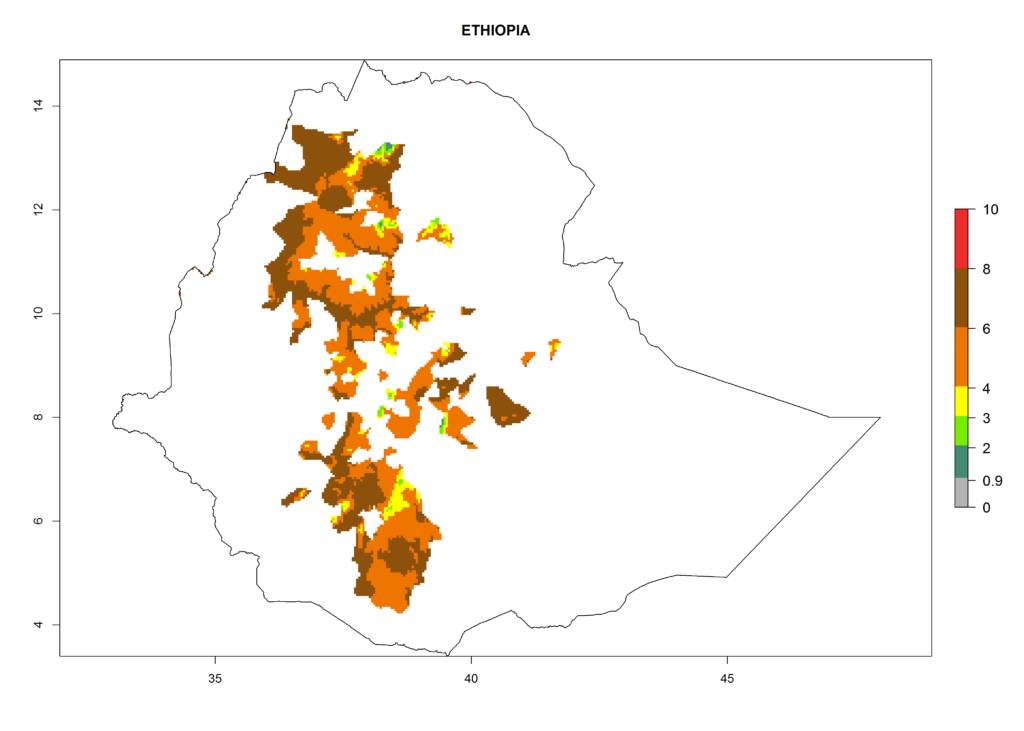 |
 |
| d) Kenya | 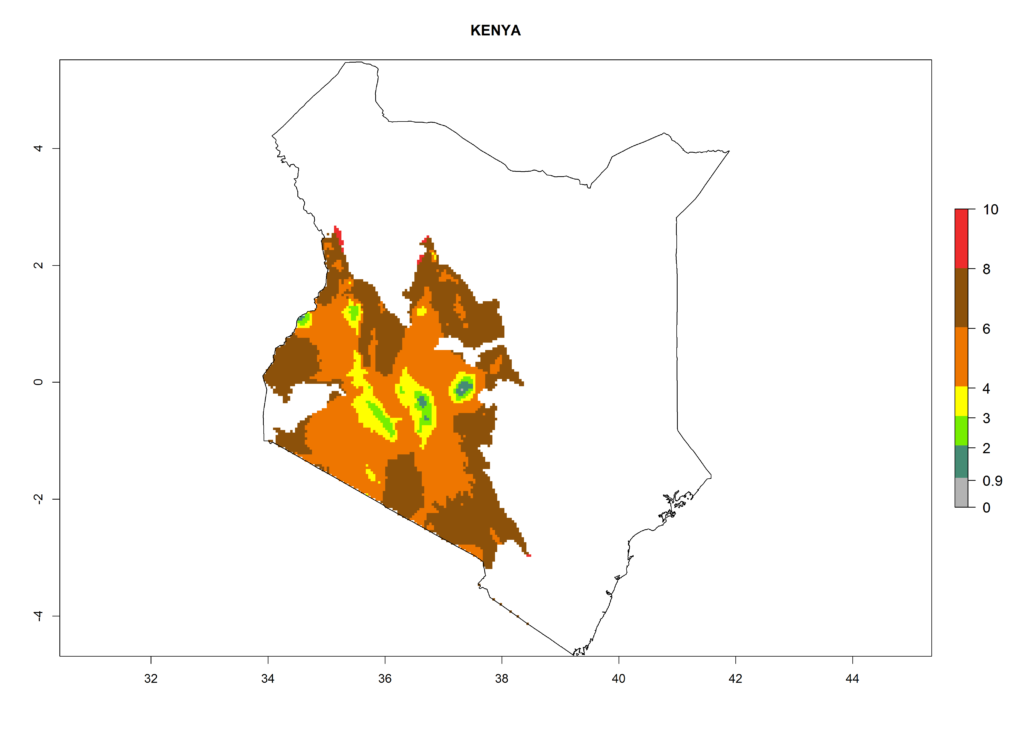 |
 |
| e) Madagascar | 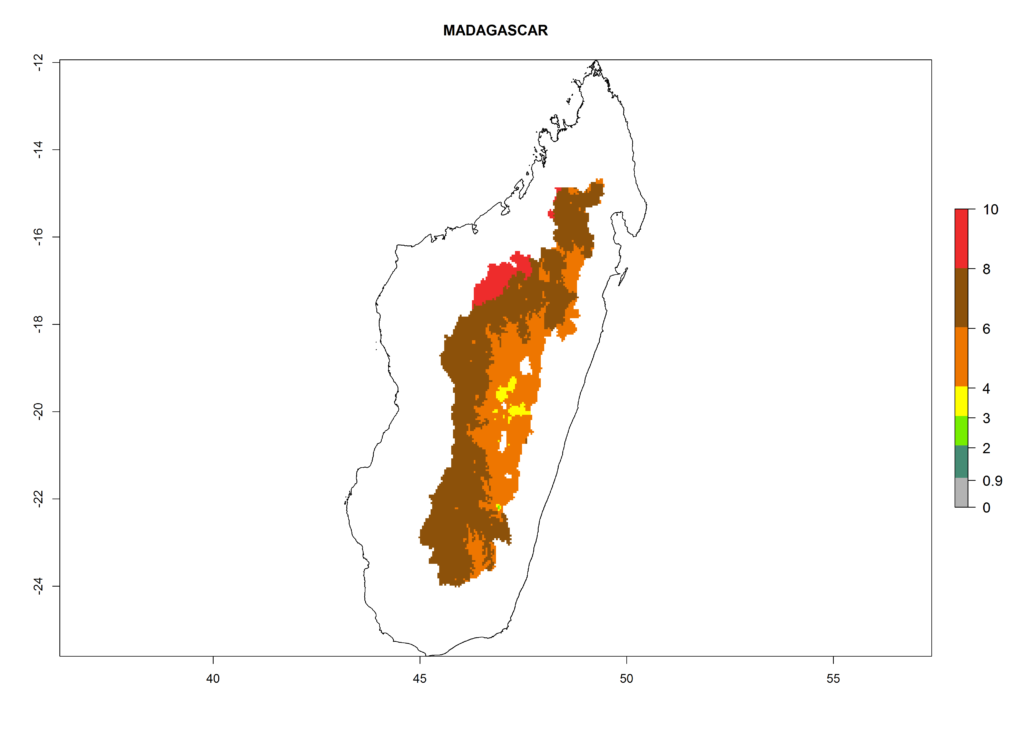 |
 |
| f) Malawi | 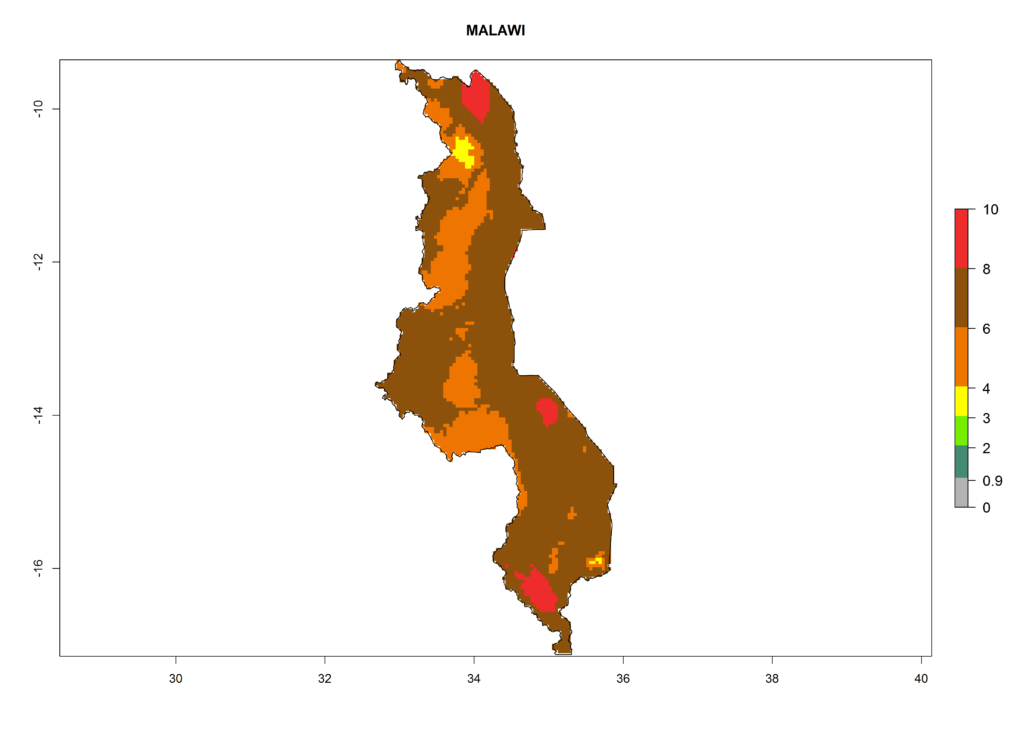 |
 |
| g) Morocco | 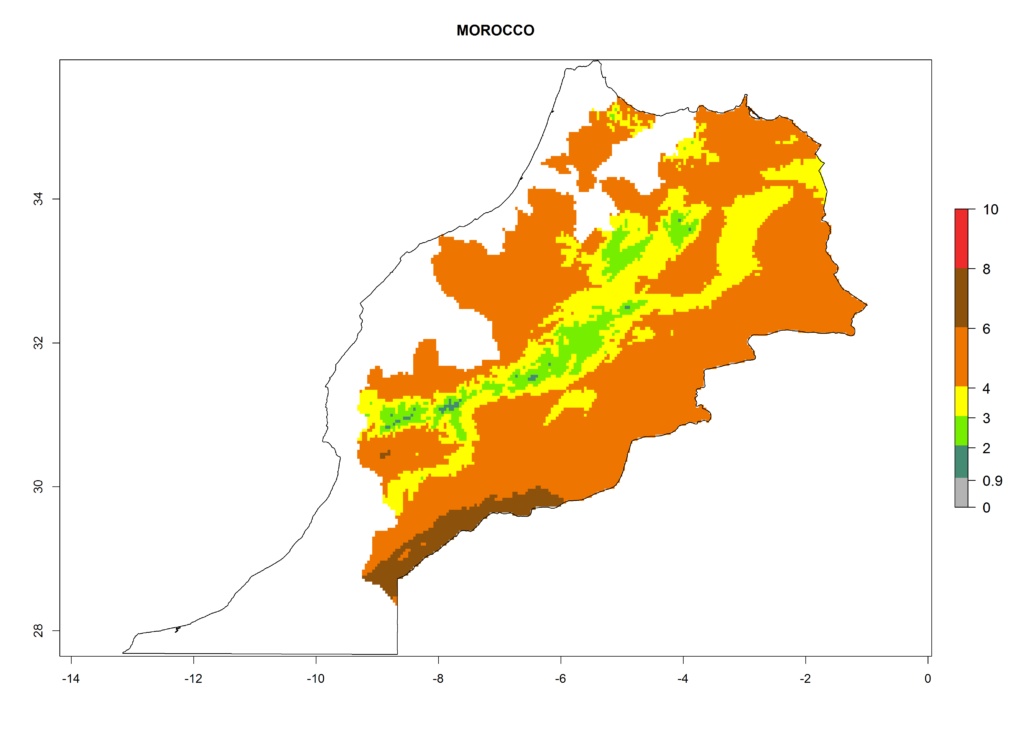 |
 |
| h) Nigeria | 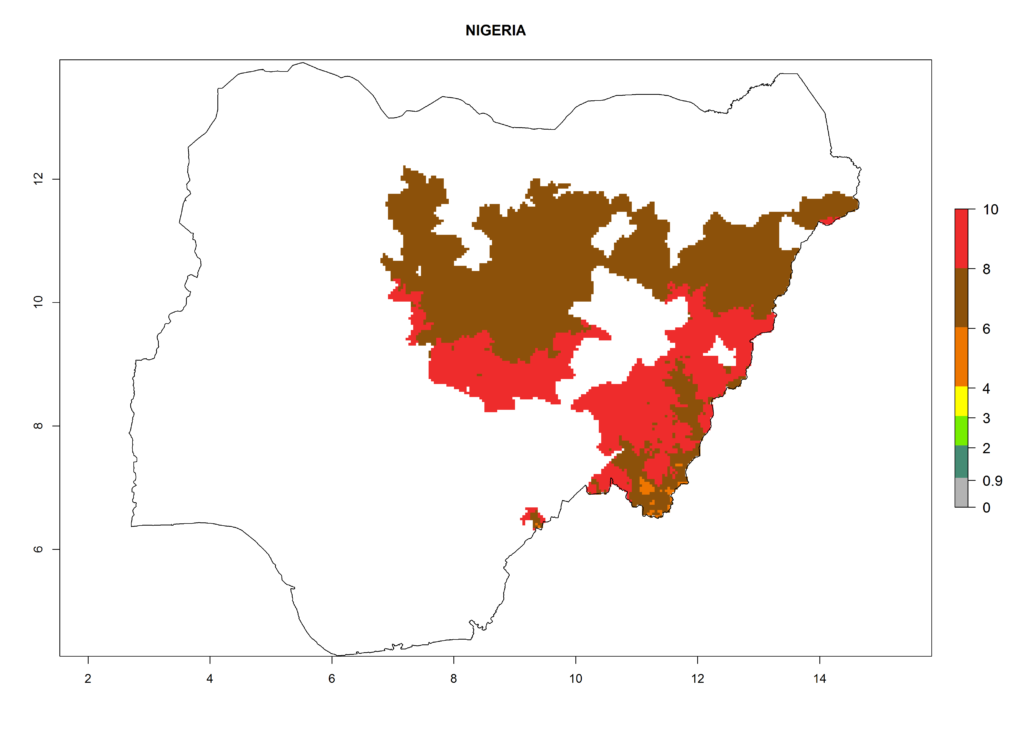 |
 |
| i) Rwanda | 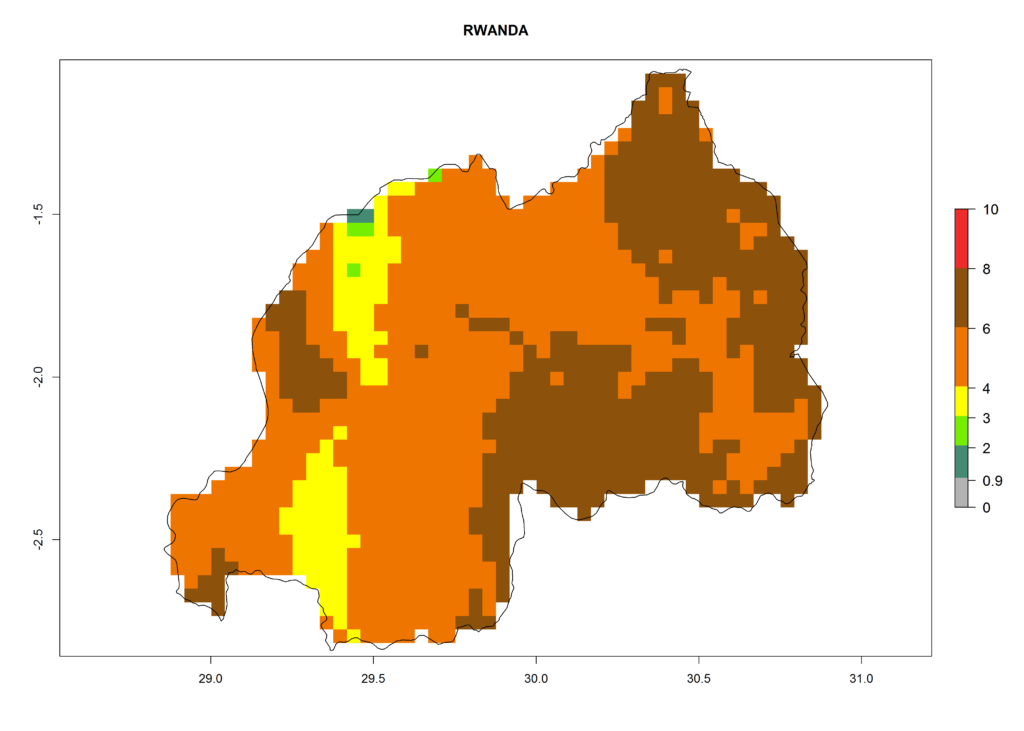 |
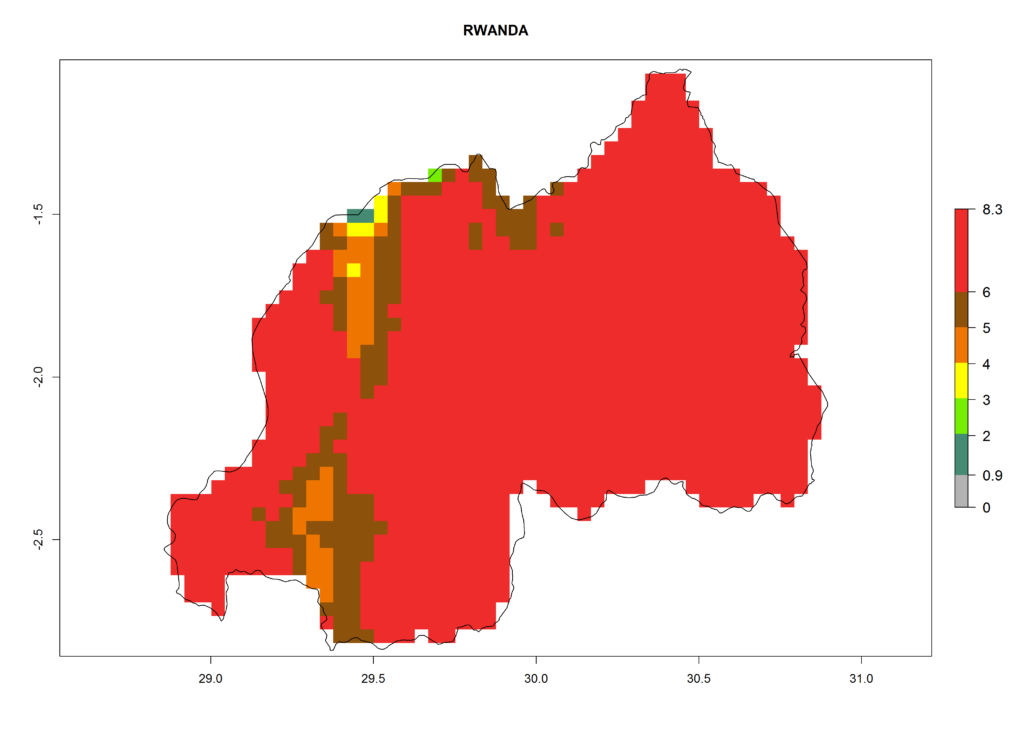 |
| j) Tanzania | 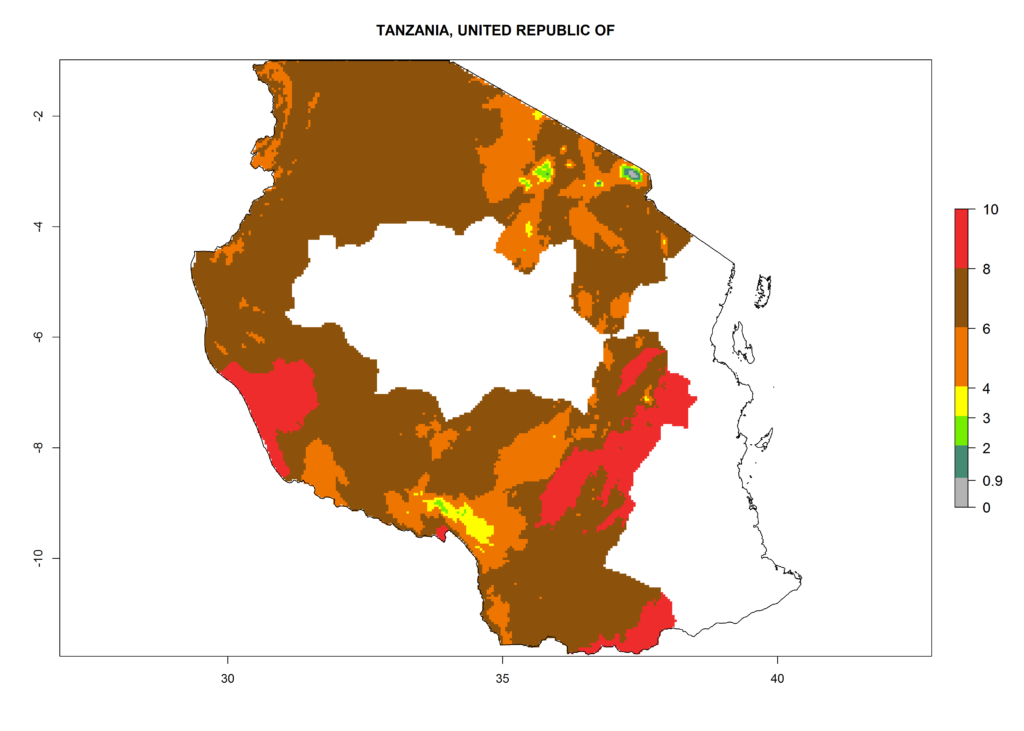 |
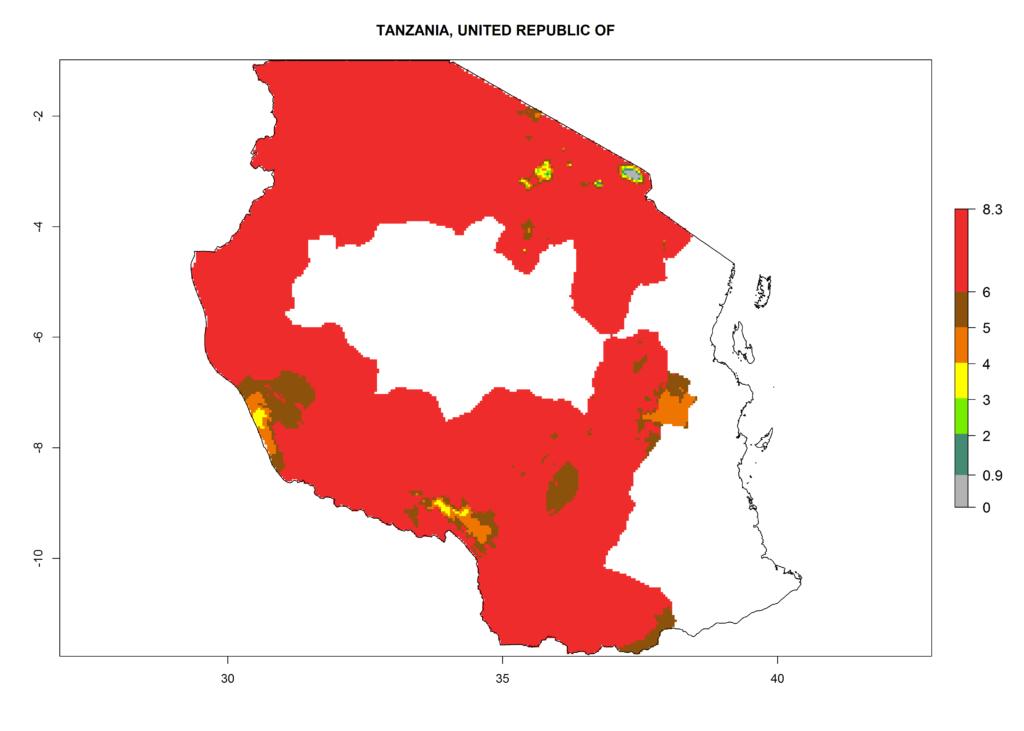 |
| k) Tunisia | 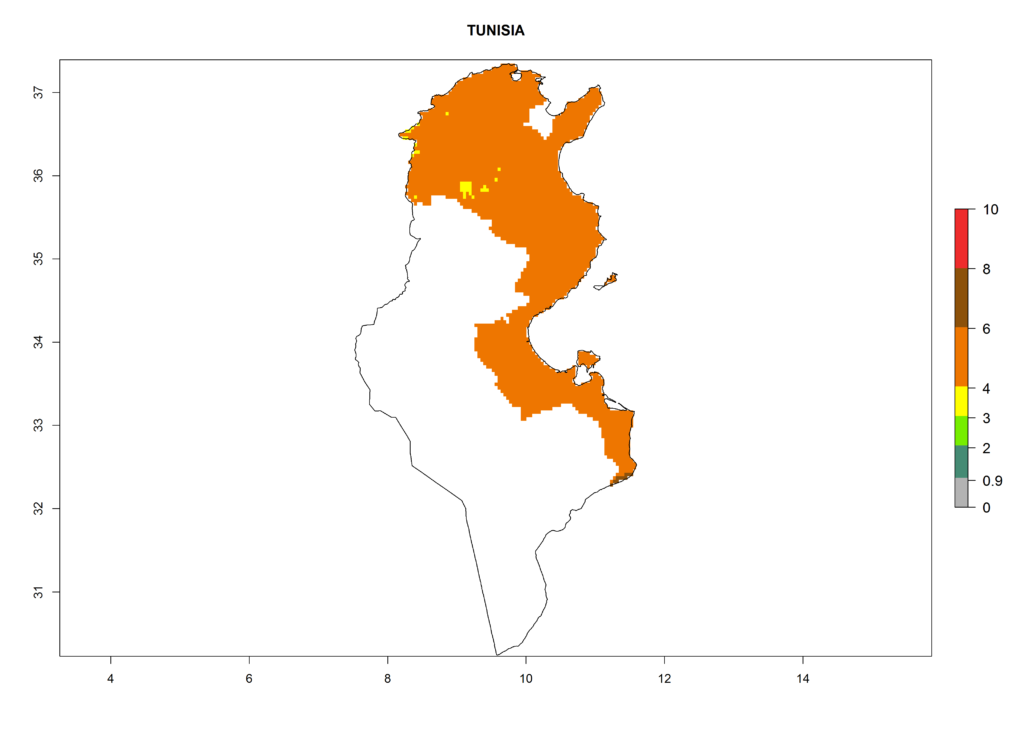 |
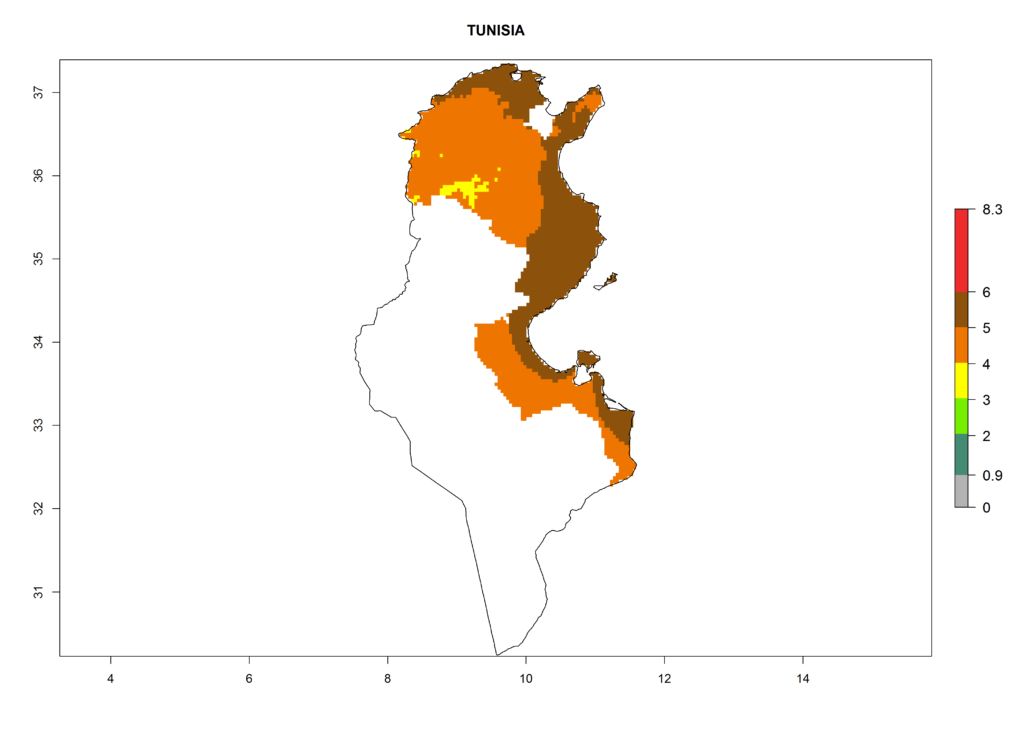 |
| l) Uganda | 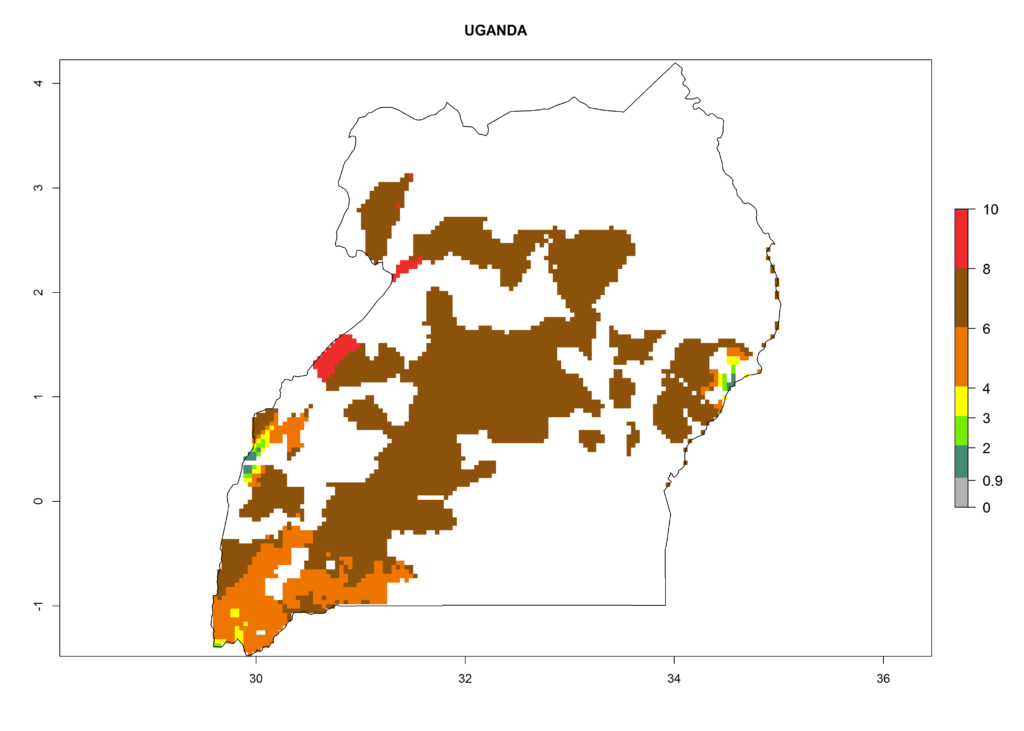 |
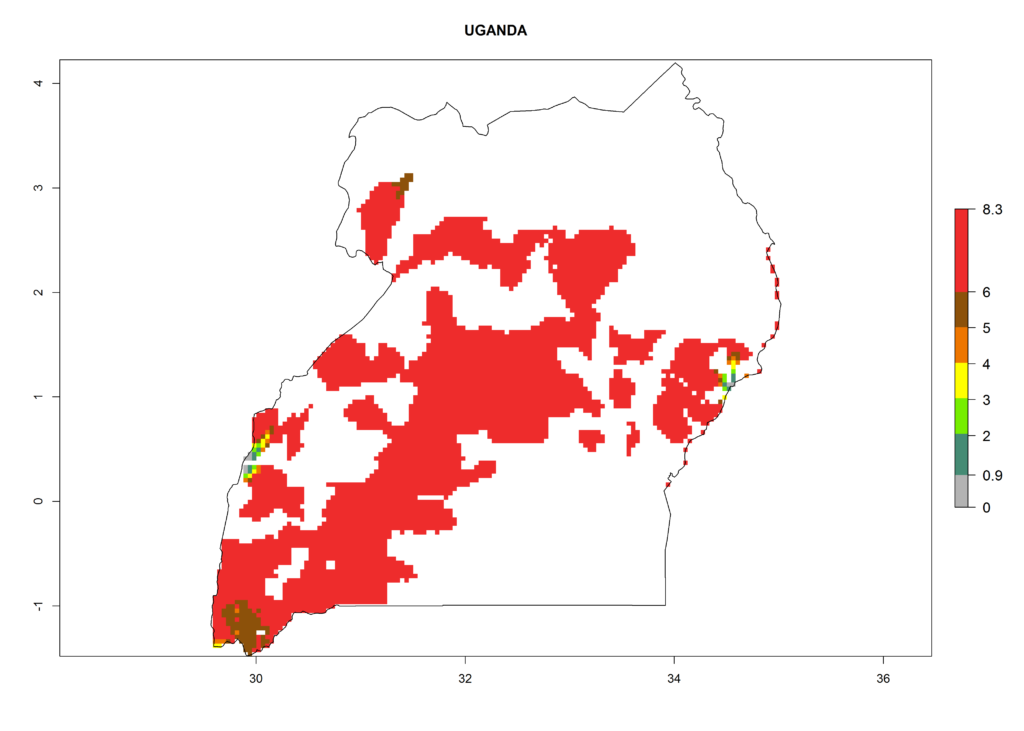 |
Figure 6. ERI, abundance (GI, damage potential), and activity (AI, potential population growth) of the Andean potato tuber moth, Symmetrischema tangolias, in potato production regions of selected African countries according to model predictions for the year 2000. An ERI>0.95 is associated with potential permanent establishment.
Phytosanitary measures
Infestation of potato tubers with eggs or young larvae of S. tangolias is hard to detect. Only a strict ban on importation of potato tubers from countries where the pest is present can prevent the dissemination effectively. General recommendations are importation of clean, soil-free potatoes, cooling during shipping, and the use of new or disinfected packing materials from potential source countries. Ethiopia, which has already listed S. tangolias as an arthropod pest associated with the pathway of importing potato tubers into the country from major source countries (in this case, from Peru), may require phytosanitary measures. Initially, a permit to import planting material from Peru is requested. External inspection of tubers is, however, insufficient to fully confirm non-infestation. Dissection and/or incubation methods could provide adequate information, although such methods are time consuming.
Adaptation to risk avoidance at farm level
Since S. tangolias is still not a pest in any African country, the most important issue is to prevent its introduction into the continent. The integrated pest management components are similar to those for the common potato tuber moth, P. operculella (for these strategies, see section 4.1.1, P. operculella), with the exception that the granulovirus attacking P. operculella is not effective against S. tangolias. S. tangolias is difficult to control as the larvae feed inside potato tubers during the storage period where they are hard to reach. The cycle continues in growing potato crops that makes it difficult to break the pest’s life cycle. Therefore, effective management of S. tangolias needs to focus on both prevention of storage infestation and controlling the pest in the field; this is most successful when a range of management methods are applied.
Crop and Field Management
Monitoring with pheromone traps. Sex pheromone-baited water or funnel (delta) traps indicate the presence of S. tangolias in the field and store by attracting male adults. They can be used to detect the early presence of S. tangolias in order to take adequate control.
Classical biological control. Classical biological control can be an effective strategy in all those regions where the pest has been unintentionally introduced to keep the pest population under the control threshold (described in section 4.1.1, P. operculella).
Avoid infestation of pest-free potato-growing zones. Infested seed facilitates the fast spread of the pest and is a prime source of initial infestation in potato fields. Thus, pest-free seed tubers need to be planted.
Attract-and-kill. This is an oil formulation with the pest’s sexual pheromone and contact insecticide as active ingredients, which are applied at a density of 2,500 droplets (100 µl)/ha in the field to reduce the male population and hence leaf and tuber infestation.
Adequate hilling. In the field, larvae need to enter the soil to reach the potato tubers. The infestation of potato tubers decreases with the depth in soil where they develop. Therefore potato tubers need to be covered well with soil during the whole cultivation period.
Avoid dry soil conditions. Dry weather conditions favor multiplication of S. tangolias in the field as the number of eggs laid decreases strongly with higher soil moisture; larvae drown in highly moist soil as well. The sowing date should be adjusted to avoid long periods of dry weather, especially in the weeks before harvest. If there is modest rain, sprinkler irrigation can be used to moisten the soil.
Timely and complete harvest. High tuber damage at harvest has been frequently caused by delayed harvests in combination with dry weather conditions that enable several generations to develop in the field. During dry periods, timely harvest is crucial to avoid tuber infestation (the larvae move from the stems into the tubers when the plant wilts). Early-maturing varieties can contribute to reduce the risk of infestation. Leftover tubers (volunteer plants) are frequently infested by S. tangolias, providing a source of re-infestation and need to be removed from the field.
Storage Management
Clean storage facilities. Infested potato stores are a source of contamination for tubers stored after harvest and in surrounding fields and stores. Before potato tubers are stored, the stores need to be cleaned thoroughly. Eggs, larvae, and pupae frequently found in cracks of the walls, the soil, or in bags or boxes used for storage need to be removed. Only healthy tubers should be selected for storage.
Biological control. In Peru, a dust formulation of Bacillus thuringiensis is used to protect tubers in storage against S. tangolias. B. thuringiensis var. kurstaki is effective against S. tangolias. The abrasive effect of different kinds of powders (kaolin, calcium carbonate, or others), which is the basis of the formulation, also kills young larvae due to the physical protective capacity of the powder. Potato tubers should be treated (powdered) directly after harvest and placed in storage.
Attract-and-kill can be applied at a density of one drop (100 µl)/m2 of storage area to reduce the male population and hence tuber infestation.
Further reading
Dangles, O., M. Herrera, and F. Anthelme. 2013. Experimental support of the stress-gradient hypothesis in herbivore-herbivore interactions. New Pathologist 197: 405–408.
Dangles, O., M. Herrera, C. Mazoyer, and J.-F. Silvain. 2013. Temperature-dependent shifts in herbivore performance and interactions drive nonlinear changes in crop damages. Global Change Biology 19: 1056–1063.
Dangles, O., V. Mesías, V. Crespo-Perez, and J.-F. Silvain. 2009. Crop damage increases with pest species diversity: evidence from potato tuber moths in the tropical Andes. Journal of Applied Ecology 46: 1115–1121.
Dangles, O., C. Carpio, A. Barragan, J.-L. Zeddam, and J.-F. Silvain. 2008. Temperature as a key driver of ecological sorting among invasive pest species in the tropical Andes. Ecological Applications 18: 1795–1809.
Ewell, P.T., H. Fano, K.V. Raman, J. Alcázar, M. Palacios, and J. Carhuamaca. 1990. Farmer management of potato insect pests in Peru. 77 pp., Food Systems Research Series, International Potato Center (CIP), Lima, Peru.
Griepink, F.C., F.P. Drijfhout, T.A. v. Beek, J.H. Visser, and A. d. Groot. 2000. Analysis of sex pheromone gland content of individual Symmetrischema tangolias by means of direct gland introduction into a two-dimensional gas chromatograph. Journal of Chemical Ecology 26: 1013–1023.
Griepink, F.C., T.A. v. Beek, J.H. Visser, S. Voerman, and A. d. Groot. 1995. Isolation and identification of sex pheromone of Symmetrischema tangolias (Gyen) (Lepidoptera: Gelechiidae). Journal of Chemical Ecology 21: 2003–2013.
Keller, S. 2003. Integrated pest management of the potato tuber moth in cropping systems of different agro-ecological zones. In J. Kroschel (ed.) Tropical Agriculture 11, Advances in Crop Research 1, 163pp. Weikersheim, Germany: Margraf Publishers.
Kraan, C. v. d., and P. v. Deventer. 1990. Pheromone of Symmetrischema plaesiosema (Turner) (Lep., Gelechiidae): a 3-component system with notable promise for communication disruption. Journal of Applied Entomology 109: 213–215.
Kroschel, J., and B. Schaub. 2013. Biology and ecology of potato tuber moths as major pests of potato. In Insect pests of potato—Global perspectives on biology and management, ed. A. Alyokhin, C. Vincent, and P. Giordanengo, 165–192. Waltham, MA: Elsevier.
Kroschel, J., and O. Zegarra. 2010. Attract‐and‐kill: a new strategy for the management of the potato tuber moths Phthorimaea operculella (Zeller) and Symmetrischema tangolias (Gyen) in potato: laboratory experiments towards optimizing pheromone and insecticide concentration. Pest Management Science 66(5): 490–496.
Kroschel, J., and O. Zegarra. 2013. Attract‐and‐kill as a new strategy for the management of the potato tuber moths Phthorimaea operculella (Zeller) and Symmetrischema tangolias (Gyen) in potato: evaluation of its efficacy under potato field and storage conditions. Pest Management Science 69(11): 1205–1215.
Kroschel, J., N. Mujica, J. Alcazar, V. Cañedo, and O. Zegarra. 2012. Developing integrated pest management for potato: experiences and lessons from two distinct potato production systems of Peru. In: Sustainable Potato Production: Global Case Studies, 419–450. Springer, Netherlands.
Lagnaoui, A., V. Cañedo, and D. S. Douches. 2001. Evaluation of Bt-cry1la1 (cryV) transgenic potatoes on two species of potato tuber moth, Phthorimaea operculella and Symmetrischema tangolias (Lepidoptera: Gelechiidae) in Peru, pp. 117–121, Scientist and farmer: partners in research for the 21st Century. Program Report 1999–2000. International Potato Center (CIP), Lima, Peru.
Larraín, S.P. 2002. Insect and mite pest incidence on sweet pepinos (Solanum muricatum Ait.) cultivated in the IV Region, Chile. Agricultura Técnica 62: 15–26.
Martin, N.A. 1999. Arthropods and molluscs associated with poroporo (Solanum aviculare and S. laciniatum): an annotated species list. Journal of the Royal Society of New Zealand 29: 65–76.
Martin, N.A., P.J. Workman, and T. Marais. 1996. IPM for greenhouse crops in New Zealand: progress, problems and prospects. Bulletin OILB/SROP 19: 99–102.
Ortiz, O., J. Kroschel, J. Alcázar, R. Orrego, and W. Pradel. 2009. Evaluating dissemination and impact of IPM: lessons from case studies of potato and sweetpotato IPM in Peru and other Latin American countries. In Integrated pest management: Dissemination and impact (pp. 419–434). Springer, Netherlands.
Palacios, M., J. Tenorio, M. Vera, F. Zevallos, and A. Lagnaoui. 1999. Population dynamics of the Andean potato tuber moth, Symmetrischema tangolias (Gyen), in three different agro-ecosystems in Peru. pp. 153–160, Impact on a changing world. International Potato Center Program Report 1997–1998. International Potato Center (CIP), Lima, Peru.
Povolny, D. 1994. Gnorimoschemini of southern South America VI: identification keys, checklist of Neotropical taxa and general considerations (Insecta, Lepidoptera, Gelechiidae). Steenstrupia 20: 1–42.
Ragoussis, V., S. Perdikaris, A. Karamolegkos, and K. Magkiosi. 2008. Improved synthesis of (3 E, 7 Z)-3, 7-Tetradecadienyl Acetate, the major sex pheromone constituent of the potato pest Symmetrischema tangolias (Gyen). Journal of Agricultural and Food Chemistry 56(24): 11929–11932.
Raman, K.V. 1988. Integrated insect pest management for potatoes in developing countries. CIP Circular, International Potato Center 16: 1–8.
Suckling, D.M., J.T.S. Walker, G.K. Clare, K.S.H.B. Wilson, C. Hall, A.M. El-Sayed, and P.S. Stevens. 2012. Development and commercialization of pheromone products in New Zealand. New Zealand Plant Protection 65: 267–273.
Wale, S.J., H. (Bud) Platt, and N. Cattlin, eds. 2008. Diseases, Pests and Disorders of Potatoes: A Colour Handbook. London, UK: Manson Publishing Ltd, 176pp.