4.2.4 Sweetpotato Pests / Sweetpotato white fly, Bemisia tabaci (Gennadius 1989) (Biotype B)![]()
Synonyms: B. inconspicua (Quaintance 1900–Aleurodes)
B. emiliae (Corbett 1926)
B. signata (Bondar 1928)
B. bahiana (Bondar 1928)
B. costa-limai (Bondar 1928)
B. gossypiperda (Misra & Lamba 1929)
B. chyranthes (Singh 1931)
B. hibisci (Takahashi 1933)
B. longispina (Priesner & Hosny 1934)
B. gossypiperda var. mosaicivectura (Ghesquiere 1934)
B. goldingi (Corbett 1935)
B. nigeriensis (Corbett 1935)
B. rhodesiaensis (Corbett 1936)
B. manihotis (Frappa 1938)
B. vayssieri (Frappa 1939)
B. lonicerae (Takahashi 1957)
B. minima (Danzig 1964)
B. minuscula (Danzig 1964)
A complete list of synonyms is given by Mound and Halsey (1978) and Perring (2001).
Taxonomic position: Insecta, Hemiptera, Aleyrodidae
Authors: H. Gamarra, N. Mujica, P. Carhuapoma, J. Kreuze, & J. Kroschel
Common names
Tobacco whitefly, sweetpotato whitefly, cotton whitefly, cassava whitefly, silverleaf whitefly, the B. tabaci ‘B-biotype/strain’, ‘poinsettia strain’ (English), Aleurode du cotonnier, aleurode de la patate douce (French), Weisse Fliege, Baumwollmottenschildlaus, Tabakmottenschildlaus (German), Mosca blanca del algodonero, mosca blanca del tabaco, mosca blanca del camote, (Spanish), Aleirode delle solanacee, aleurode delle solanacee (Italian), Mosca branca do feijao (Portuguese)
Hosts
Sweetpotato [Ipomoea batatas (L.) Lam.], cotton (Gossipum barbadense L.), beans (Phaseolus vulgaris L.), tomato (Solanum lycopersicum L.), cucumber (Cucumis sativus L.), melon (Cucumis melo L.), soya (Glyxine max L Merrill), paprika (Capsicum annum L.), cassava (Manihot esculenta Crantz), lettuce (Lactuca sativa L.), grape (Vitis vinifera L.), pumpkin (Cucurbita maxima L.), watermelon (Citrullus lanatus (Thunb.) Matsum. et Nakai), cabbage (Brassica oleracea L. var. capitata L.), eggplant (Solanum melongena L), avocado (Persea americana Mill.), annona (Annona muricata L.), broccoli (Brassica oleracea L.), Chinese cabbage (Brassica rapa L.), ficus (Ficus benjamina L.), guava (Psidium guajava L.), lantana (Lantana camara L.), potato (Solanum tuberosum L.), sunflower (Helianthus annus L.), bean (Vicia faba L.); ornamental plants: geranium (Pelargonium graveolens L’Her.), chrysanthemum (Chrysanthemun sp.), hibiscus (Hibiscus rosa-sinensis L.), and others.
Detection and identification
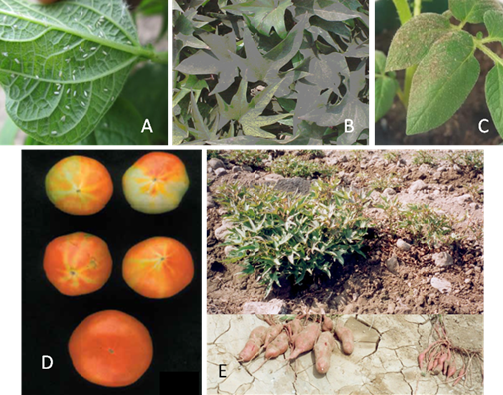
Adults and nymphs of Bemisia tabaci cause three types of damage to plants. First, direct suction of plant sap: damage by sucking sap from plant tissue is associated with several physiological plant disorders such as irregular ripening or incomplete fruit coloration of tomato that results in streaking (Photo 1D); severe pod chlorosis of bean, squash silverleaf in many Cucurbita species (Cucurbita pepo, C. moschata, and C. mixta), lettuce leaf yellowing and stem blanching, carrot light root, pepper streak, Brassica white stem, and chlorosis of new foliage in many plants. Second, honeydew covering plants caused by extensive sugar excretions interfere with normal photosynthetic processes and allow the growth of fungi, leading to sooty mold, which in some cases can affect the quality of the harvest product (Photos 1A–C). Third, transmission of plant viruses by adults, which is the most important damage
of whiteflies. Main viruses are Begomoviruses (Geminiviridae: Begomovirus), such as the sweetpotato, tomato leaf curl viruses, and cassava and bean mosaic viruses. Viruses of the genera Crinivirus (Family: Closteroviridae) such as the sweet potato chlorotic stunt virus (Photo 1E), and tomato chlorosis viruses. Viruses of the genera Ipomovirus (Family: Potyviridae) such as cassava brown streak viruses and Sweet potato mild mottle virus; and the Carlavirus (Family: Betaflexiviridae) such as the cowpea mild mottle virus, cause symptoms of blisters and general chlorosis in bean plants. Plant symptoms do not occur often enough to confirm the presence of a particular whitefly species; therefore the use of molecular or morphological studies of the fourth instar (pupa) is necessary to confirm the identification of the species (Photo 2). Identification of B. tabacci biotypes is not possible based solely on morphological characteristics. Therefore the mtCOI marker has been used increasingly to assess the genetic variability of B. tabaci populations in Africa and elsewhere.
Morphology
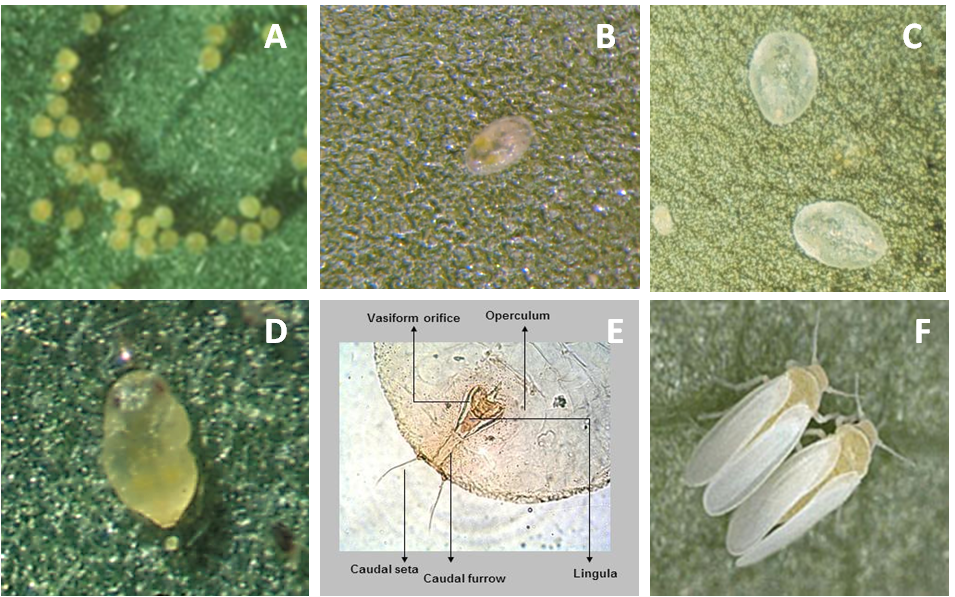
Egg
On average, eggs are 0.1 x 0.19 mm in size. Initially they are white, but as they mature turn to light orange and finally dark orange. The biotype B of B. tabaci can lay their eggs in isolation, in irregular groups, or in a semicircle (Photo 2A).
Nymphal instars
First nymphal instars (0.16 x 0.26 mm), called “crawlers,” are flat, oval, and almost transparent (Photo 2B). Second nymphal instars (0.36 x 0.24 mm) are translucent and oval with wavy edges. Third nymphal instars are 0.53 x 0.36 mm in size (Photo 2C).
Pupa (Fourth nymphal instar)
The fourth instar nymphs (0.73 x 0.45 mm) are oval, flat, and transparent (Photo 2D). As development progresses, pupa becomes bulky and opaque and has visible red eyes. At this point it is called puparium. It is heart-shaped, with rounded cephalic part and caudal part with a pointed end. In profile it protrudes high on the surface of the leaf as compared with other nymphal stages. Molecular or morphological studies of the fourth instar (pupa) are used to confirm the identification of the species (Photo 2E).
Adult
Wings of newly emerged adults (0.70 x 0.95 mm) are clear but become covered with a white wax over time. The female is larger than the male (Photo 2F).
Biology
Adults lay and fix their elongated oval eggs with the help of a peduncle on the underside of plant leaves. On hairy leaves eggs are laid randomly across the leaf surface. The nymphs have tiny legs that allow movement across the leaf surface. After hatching the nymph moves a short distance until it successfully probes the leaf and obtains plant sap, then settles down to feed. It remains at this location until it develops into an adult. Adult whiteflies emerge through a T-shaped slit in the integument of the last nymphal instar (puparium) and, after flying a few hours, adults begin feeding by piercing leaves and sucking plant sap, which continues for the rest of their life time. During the first few days, adults move from old leaves to younger leaves on the same or different plants. Thereafter they spend most of their time on the undersides of the topmost leaves, but will fly when disturbed. The female lives 12–63 days and feeds and lays eggs on the abaxial surface of leaves. Adults mate as soon as they emerge, but there might be a pre-oviposition period of 1 or 2 days, depending on the temperature. B. tabaci biotype B females can reproduce through parthenogenesis, giving rise to progeny exclusively of males.
Temperature-dependent development
B. tabaci biotype B completed its development from egg to adult in all temperatures at 15°–32°C, but not at 10°–12ºC and 35°C (see Annex 7.3.7). Whitefly total development was almost four times longer at 15ºC (156.54 days) than at 32ºC (35.73 days). Estimated lower threshold for immature stages were 10.5ºC (egg), 14.19ºC (nymph), and 14.6ºC (pupa). For all three immature stages, lowest mortality occurred at 20ºC (2.4%) and the highest at 15ºC (56.3%). The pupa was more vulnerable to unfavorable temperatures compared with other stages. Optimal temperatures for survival were 20°–25ºC (79–90% survival of the entire immature stage). Significant differences
were observed in the longevity between male and female. Females lived twice as long as males at temperatures >15ºC. Lowest senescence rate was observed within the temperature range of 18°–20°C. Highest fecundity is found at around 25ºC (368 eggs/female).
The established functions (see Annex 7.3.7) were used to estimate the life-table parameters of B. tabaci biotype B and build an overall deterministic phenology model. Population parameters indicate that the intrinsic rate of natural increase (rm) augments almost linearly with increasing temperature to reach a maximum of 27ºC (0.148) and then decreased sharply at 30ºC (0.089). Similarly, finite rate of increase for the whitefly peaked at 27ºC (λ=1.159) and was smallest when exposed to 15ºC (λ=1.007) and 32ºC (λ=1.004). The highest values for both reproductive parameters (gross reproductive rate and net reproductive rate, Ro) were 22.5ºC. The shortest mean generation time (T) and doubling time (Dt) were observed at 30ºC (23.55 days) and 27ºC (4.674 days), respectively. The optimum temperature for overall population growth ranged 20º– 25ºC.
Deterministic simulation of life-table parameters under temperature conditions of lowland agro-ecologies at the central coast of Peru (Lima, La Molina: 12º 05′ S, 76º 57′ W, 250 masl, with a mean annual precipitation of 6.4 mm and mean temperature of 19.7ºC) showed that the whitefly population increases in all seasons (λ>1). Simulated T for each season predicted that B. tabaci can develop 2.5 generations in summer (mean temperature of 23.3ºC), 1.9 generations in autumn (mean temperature of 18.9ºC), 2.0 generations in winter (mean temperature of 16.8ºC), and 2.2 generations in spring (mean temperature of 19.7ºC); with a total of 8.6 generation per year.
Means of movement and dispersal
Adults may simply fly up the same plant or over to neighboring plants; short-distance migration of up to several kilometers may also occur. Migrating individuals usually develop on plants that are senescing. Migrations can be massive and lead to severe infestation of newly planted crops. Dispersal over long distances is facilitated by wind currents and through the exchange of planting material between regions, countries, and continents.
Economic impact
B. tabaci biotype B is one of the most important pests worldwide due to its wide distribution in tropical and subtropical regions. It can affect more than 600 different species of cultivated and wild plants and, due to the large number of viruses it can transmit, particularly Begomoviruses (Geminiviridae: Begomovirus), can cause devastating disease in many different crops. Sweet potato leaf curl viruses can cause losses of up to 30%, and cassava mosaic Begomoviruses are the most serious threat to cassava production in Africa and some parts of Asia. Bean mosaic Begomoviruses are the two most widely distributed pathogens in Latin America, where they cause yield losses of up to 100%. Tomato leaf curl Begomoviruses are among the most devastating diseases of tomato, causing economic losses up to 100% in many tropical and subtropical regions, and are spreading toward new areas. Ipomoviruses are also transmitted by this insect and include the causal agents of serious vein-yellowing diseases of cucurbits, the current epidemic of cassava brown streak disease, but also sweetpotato and tomato mild mottle virus. For the genus Crinivirus (Family: Closteroviridae), Sweet potato chlorotic stunt virus is the most important virus affecting sweetpotato due to its ability to mediate severe synergistic diseases with several other sweetpotato-infecting viruses belonging to different genera. The virus causes losses of up to 100%; tomato chlorosis virus is also of worldwide concern.
Geographical distribution
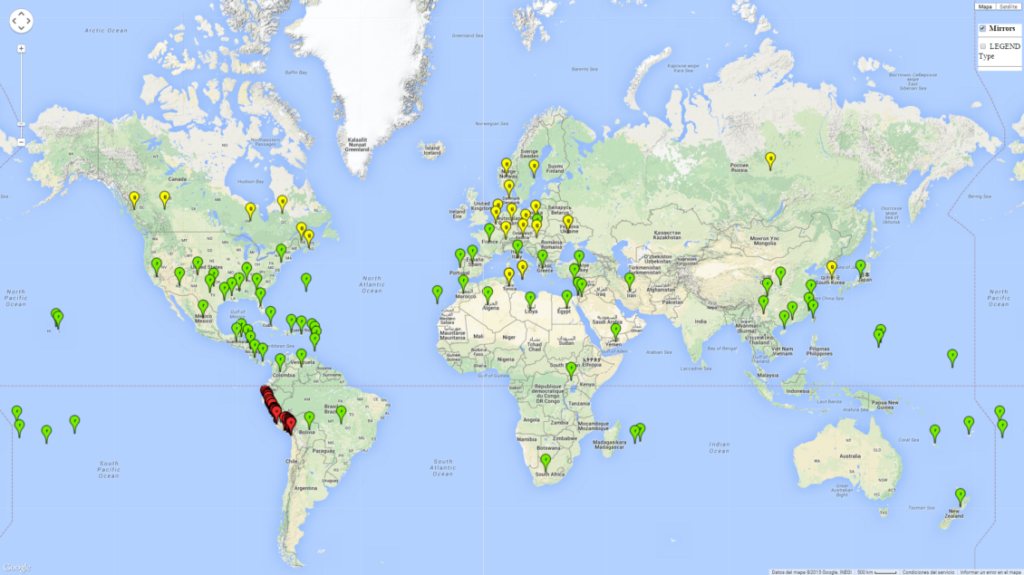
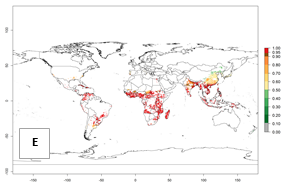
Currently the biotype B of B. tabaci has become a global pest in tropical and subtropical regions, and is less prominent in temperate habitats (Fig. 1). Environments with cold temperatures often lead to high mortality of both adult and larvae. Biotype B of B. tabaci is the most extensively studied white fly species in recent years due to its invasiveness and broad host range that has resulted in wide geographic distribution. In Europe it has successfully established in protected crops.
| Africa | Algeria, Egypt, Mauritius, Reunion, South Africa, Tunisia (greenhouse), Uganda |
| Asia | China (Fujian, Guangdong, Hainan, Sichuan, Shaanxi, Yunnan, Zhejiang), Iran, Israel, Japan, Jordan, Korea Republic (greenhouse), Taiwan, Yemen |
| Europe | Cyprus, France (South), Greece (restricted distribution), Israel, Italy (including Sardinia and Sicily), Libya, Malta, Morocco, Portugal (Algarve, Alentejo), Slovakia, Spain (including Canary Islands), Turkey and Ukraine; of limited distribution and confined almost totally to glasshouse crops in Austria, Belgium, Czech Republic, Denmark, France (Central and North), Germany, Hungary, Malta, the Netherlands, Norway, Poland, Russia, Sweden, Switzerland, Tunisia and Ukraine. In Denmark, Germany, and the Netherlands, eradication programs are set in place. |
| North America | Bermuda, Canada (only glasshouse: Alberta, British Columbia, New Brunswick, Nova Scotia, Ontario, Quebec), USA (California, Texas, Arizona, Florida, Georgia, Mississippi, Louisiana, Hawaii and its islands of Hawaii and Maui, Tennessee) |
| Central America and the Caribbean | Antigua, Barbados, Belize, Costa Rica, Cuba, Dominican Republic, Grenada, Guadeloupe, Guatemala, Honduras, Martinique, Mexico (Guanajuato, Jalisco, and San Luis Potosí), Nicaragua, Panama, Puerto Rico, St. Kitts-Nevis, Trinidad and Tobago |
| South America | Brazil (Bahia: Itaberaba, Iacú, Lençóis, Juazeiro, Guanambí, Barreiras, Maranhao, Goiad, Sau Paulo, Parana, Rio de Janeiro, and Minas Gerais), Bolivia, Colombia, Peru, Venezuela (Estado de Lara, Aragua) |
| Oceania | American Samoa, Cook Islands, Fiji, French Polynesia, Guam, Marshall Islands, New Caledonia, New Zealand, Niue, Northern Mariana Islands |
Figure 1. Global geographical distribution of the sweetpotato whitefly, Bemisia tabaci, biotype B. Green points indicate countries with reported occurrences; yellow points are countries with reported occurrence in protected crops (greenhouses); and red points are georeferenced distribution data.
Phytosanitary risks
B.tabaci biotype B is widely present globally and is not strongly considered as a quarantine pest. However, in Europe B. tabaci is listed as an A2 quarantine pest (OEPP/EPPO 1989). The risk to the European Plant Protection Organisation (EPPO) region is primarily to the glasshouse industry in northern countries and mainly concerns the biotype B (though it is difficult in practice to confirm this in specific cases). Since its recent introduction to several of these countries, the pest has proved particularly difficult to control because of its polyphagous feeding habit, its resistance to many insecticides, and limited availability of biological control methods. Very few countries remain free of B. tabaci, illustrating the difficulty of preventing its movement in international trade. Furthermore, it is likely that it is already present, but unreported, as a pest of field crops in other countries in the south of the EPPO region. In addition, the “biotype B” may now displace other biotypes on outdoor crops in southern Europe and cause much greater problems. Countries that already host many whitefly-transmitted viruses that infect indigenous plants and weeds, and are associated with localized populations of B. tabaci that exhibit narrow host ranges, may be particularly at risk from the biotype B. Should B. tabaci biotype B become established in these countries, its polyphagous feeding activities could enable viruses to move into new susceptible host plants, causing new crop protection problems.
Risks mapping under current and future climates
Global Risks
Changes in establishment and future distribution
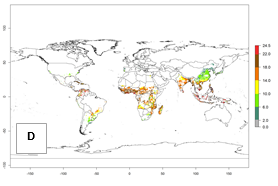
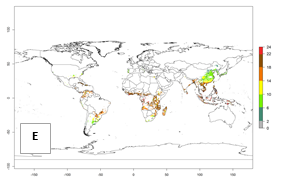
Regions with an establishment risk index (ERI)>0.95 indicate temperature conditions where a certain proportion of the biotype B of B. tabaci population is expected to survive throughout the year (Fig. 2A, D). It also reflects well the current global distribution of the pest under the climate of the year 2000.
Those regions include tropical areas of Africa, Asia, Oceania, South America, and Central America (compare with Fig. 1) where the pest is currently widely distributed. The biotype B of B. tabaci also occurs in restricted sweetpotato-growing areas with an ERI>0.7–0.9 (light- and dark-orange zones) as in southern China (Fujian, Guangdong, Guangxi, Yunnan); Southern Africa (South Africa); southern states of the United States (California, Texas, Louisiana, North Carolina); Uruguay; central Argentina; and southern Portugal (Algarve and Alentejo) (Fig. 2D). B. tabaci biotype B has also been reported in regions with a low likelihood of establishment (ERI<0.6) as in temperate regions of central and northern China, Canada, North and South Korea, Japan, northern and central Portugal, Australia, Belgium, Czech Republic, Denmark, or France (central and north). This evidence corresponds to infestations in greenhouses and not due to survival under open-field conditions (Fig. 2A, D).
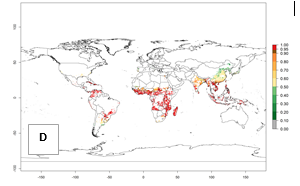
In the year 2050, a slight decrease in the risk of B. tabaci biotype B establishment is estimated (<-0.05), but the pest will continue to be a high risk in most of the tropical areas (ERI>0.95) (Fig. 2B). Moreover, it will increase with a middle-high risk (ERI>0.8) in Africa (South Africa), South America (Uruguay), and southern and central regions of China. A potential increase is also predicted for temperate regions of North America; Europe (Portugal); South America (Chile and Argentina); and Asia (northern China, South and North Korea, Japan), but with a generally low establishment potential (ERI<0.6) (Fig. 2B, C).
| ERI worldwide | ERI in sweetpotato production systems | |
| 2000 | 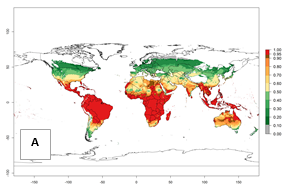 |
 |
| 2050 | 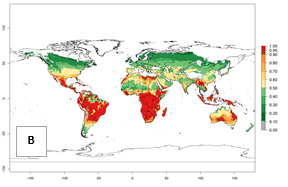 |
 |
| Index change (2000 – 2050) | 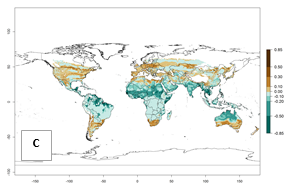 |
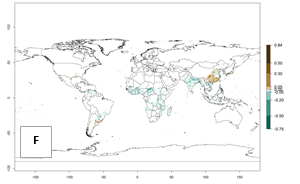 |
Figure 2. Changes in establishment and potential distribution of the sweetpotato whitefly, Bemisia tabaci biotype B, worldwide and in sweetpotato production systems according to model predictions, using the ERI for the years 2000 (A, D) and 2050 (B, E), and changes of ERI between 2000 and 2050 (C, F). An ERI>0.7 is associated with potential permanent establishment.
Changes in abundance
Under the current temperature, potentially 8–14 and 4–8 generations per year can develop in most tropical and subtropical sweetpotato-growing areas, respectively (Fig. 3A). The generation index (GI) change indicates that in most tropical and subtropical sweetpotato-growing areas, an increase of 1–3 generations is estimated (Fig. 3C). In temperate regions of South America (southern Chile) and Asia (northern China), only a slight increase of up to 1 generation per year is expected. Globally, simulated GIs gave reasonable predictions when compared with generation numbers reported in the literature (e.g., under tropical conditions, in the Middle East (mean temperatures of 12°–32°C): a mean number of 15 generations per year has been reported. Estimations made by the GI are consistent with these data.
Global maps of the activity index (AI) of B. tabaci biotype B for the year 2000 estimate a population growth potential by a factor of 18–24 in tropical regions of South America (Colombia and Venezuela), the Caribbean, West Africa, and Asia (Southeast Asia) (Fig. 3D). For most of the subtropical sweetpotato-growing areas, an AI>6–14 is predicted. Predictions of changes for the 2050 scenario indicate a small increase (AI>1–5) in the potential growth of B. tabaci biotype B in most tropical and subtropical sweetpotato-growing areas (Fig. 3E, F). Instead, increasing temperature along the equator will potentially reduce B. tabaci biotype B activity.
| GI | AI | |
| 2000 | 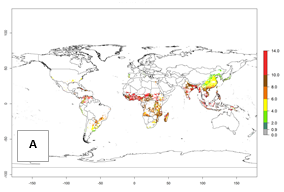 |
 |
| 2050 | 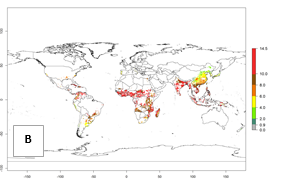 |
 |
| Index change (2000 – 2050) | 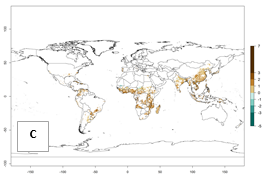 |
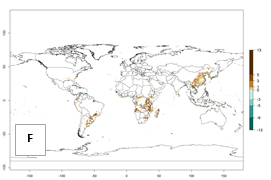 |
Figure 3. Changes in abundance (GI, damage potential) and activity (AI, potential population growth) of the sweetpotato whitefly, Bemisia tabaci biotype B, in sweetpotato production regions worldwide according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
Regional Risks for Africa
Changes in establishment and future distribution
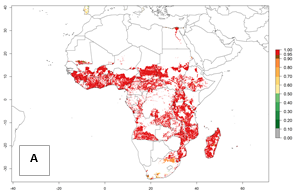
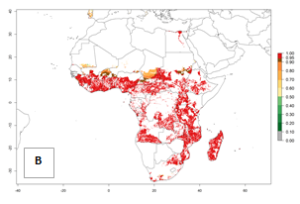
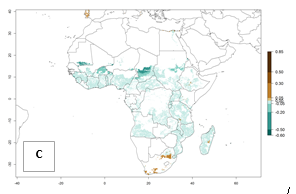
In Africa, B. tabaci biotype B represents a high risk (ERI>0.95) for all tropical and subtropical sweetpotato production areas, including northern zone of South Africa (Fig. 4A). Under the year 2050 temperature scenario, a slight decrease in the risk of establishment is estimated (<-0.05), but B. tabaci biotype B will continue to be a high risk pest (ERI>0.95) in most of the tropical and subtropical sweetpotato production regions (Fig. 4B, C).
 |
 |
 |
|
Figure 4. Changes in establishment and potential distribution of the sweetpotato whitefly, Bemisia tabaci biotype B, in African sweetpotato production systems according to model predictions, using the ERI for the years 2000 (A) and 2050 (B), and changes of the ERI between 2000 and 2050 (C). An ERI>0.7 is associated with potential permanent establishment.
Also, a high potential decrease (-0.2 to -0.5) is estimated for some areas of countries in West (Mauritania and Burkina Faso) and North-Central (Chad) Africa. By contrast, in the northern region of South Africa, the risk of establishment will strongly increase (0.1–0.3), reaching an ERI>0.95. These areas are important for the production of food crops, such as sweetpotato, which are affected by viral diseases transmitted by this species, and remain a threat to food production in the countries of eastern South Africa.
Changes in abundance
Under the current temperatures, mostly 8–13 generations per year can develop in most countries of West and North Africa, whereas fewer generations (4–10 per year) can develop in eastern, central, and southern sweetpotato-growing areas of Africa (Fig. 5A). Under the year 2050 temperature scenario, an increase of 1–3 generations per year of B. tabaci biotype B is predicted for most sub-Saharan Africa countries (Fig. 5B, C).
| GI | AI | |
| 2000 | 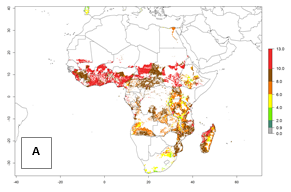 |
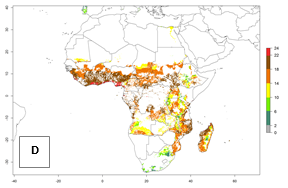 |
| 2050 | 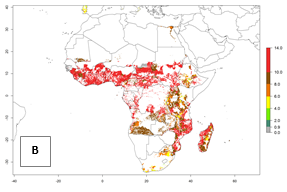 |
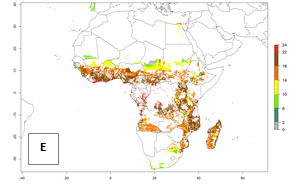 |
| Index change (2000 – 2050) | 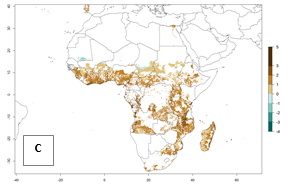 |
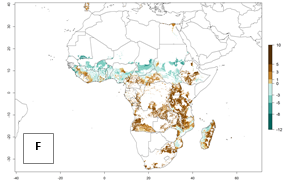 |
Figure 5. Changes in abundance (GI, damage potential) and activity (AI, potential population growth) of the sweetpotato whitefly, Bemisia tabaci biotype B, in African sweetpotato production systems according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
The AI for B. tabaci biotype B for the year 2000 estimates a population growth potential by a factor of 14–24 for most sweetpotato production regions of West and Central Africa, and by a factor of 6–18 in most countries in East and Southern Africa. An increase in the population growth potential (AI) of 1–5 is expected for most regions in East, Central, and Southern Africa (Fig. 5F). In contrast, a potential decrease (-1 to -5) is predicted for some sweetpotato-growing areas of West and North-Central Africa (Fig. 5C).
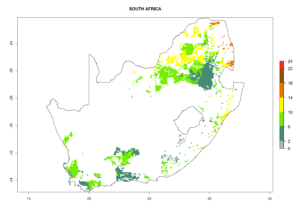
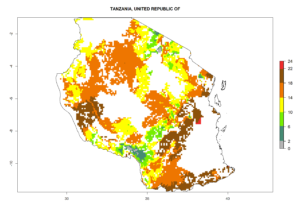
Country Risk Maps
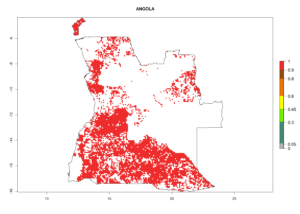
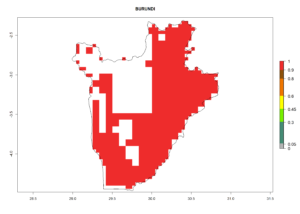
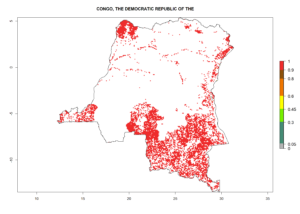
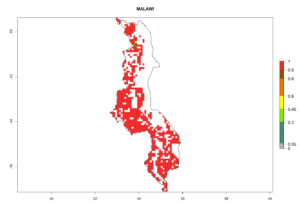
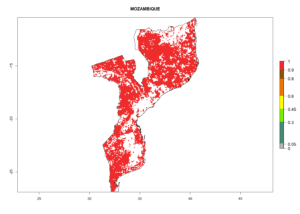
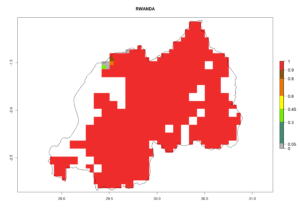
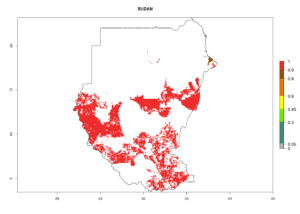
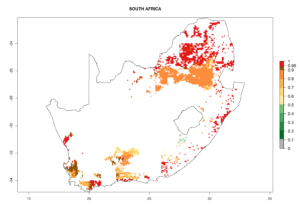
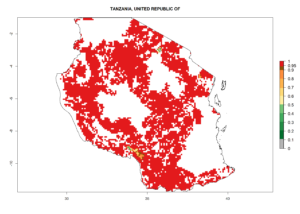
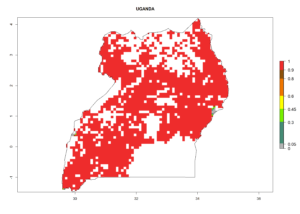
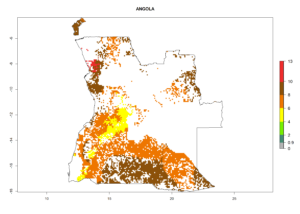
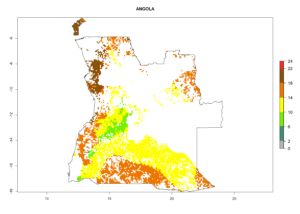
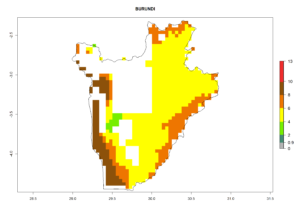
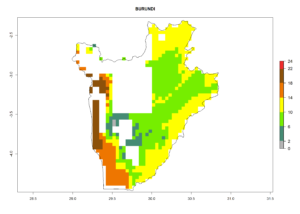
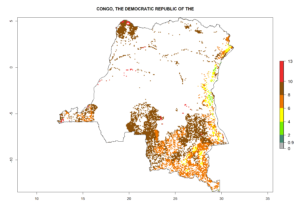
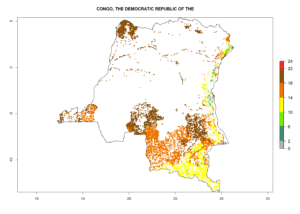
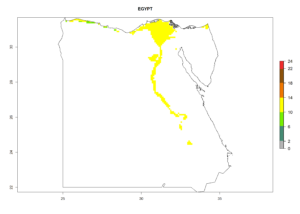
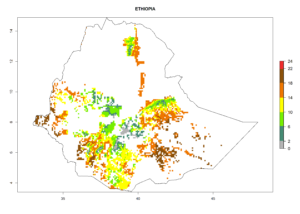
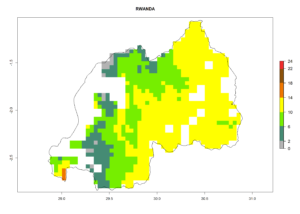
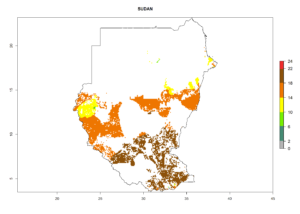
Under the current climate, B. tabaci biotype B represents a severe risk (ERI>0.95) in most sweetpotato-growing areas of countries of North (Sudan); Central (Angola and DR Congo); East (Burundi, Ethiopia, Kenya, Madagascar, Malawi, Mozambique, Rwanda, Tanzania, Uganda); and Southern (South Africa) Africa (Fig. 6). The highest potential damage (GI>8–13 generations per year) and population increase (AI>14–22) of B. tabaci biotype B are estimated for sweetpotato-growing areas of DR Congo, Madagascar, Mozambique, and Sudan (Fig. 6c, g, i, k). The lowest risks (GI>2–6 generations per year, AI>2–14) are predicted for Burundi, Rwanda, and South Africa (Fig. 6b, j, l).
| ERI | GI | AI |
| a) Angola
|
 |
 |
|
b) Burundi
|
 |
 |
| c) DR Congo
|
 |
 |
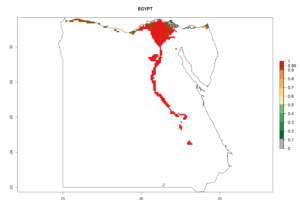
| d) Egypt
|
 |
 |
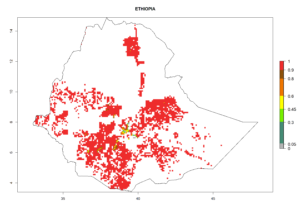
| e) Ethiopia | 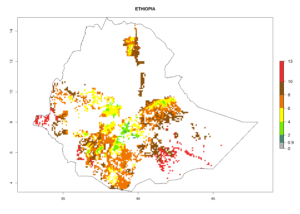 |
 |
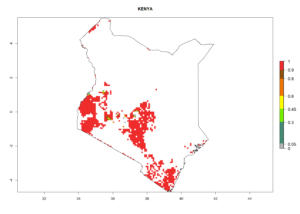
| f) Kenya | 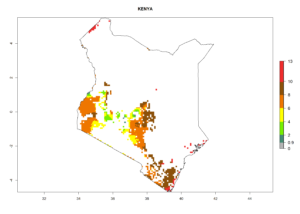 |
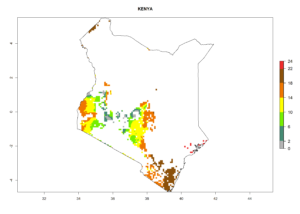 |
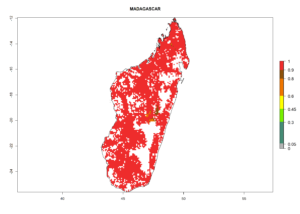
| g) Madagascar | 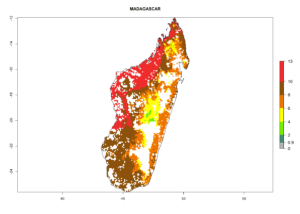 |
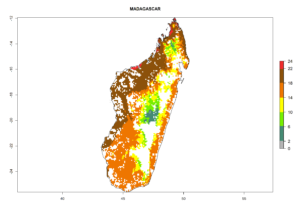 |
| h) Malawi | 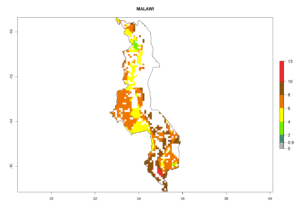 |
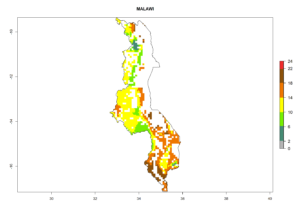 |
| i) Mozambique | 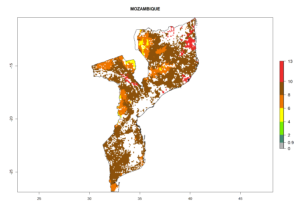 |
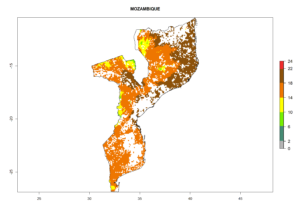 |
| J) Rwanda | 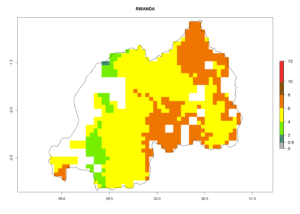 |
 |
| K) Sudan | 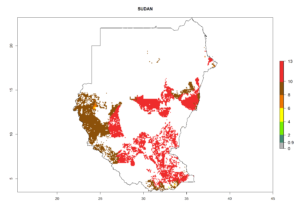 |
 |
| L) South Africa | 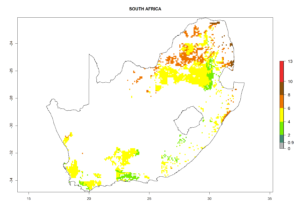 |
 |
| M) Tanzania | 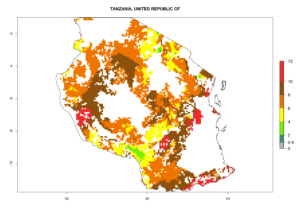 |
 |
| N) Uganda | 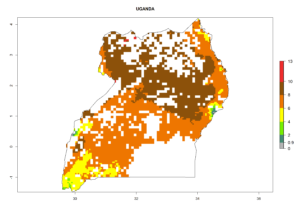 |
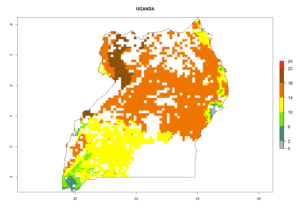 |
Figure 6. ERI, abundance (GI, potential damage), and activity (AI, potential population growth) of the sweetpotato whitefly, Bemisia tabaci biotype B, in sweetpotato production regions of selected African countries according to model predictions for the year 2000. An ERI>0.7 is associated with potential permanent establishment.
Phytosanitary measures
In countries where B. tabaci biotype B is not already present, the enforcement of strict phytosanitary regulations may help to reduce the risk of this whitefly becoming established. Because of the difficulty of detecting low levels of infestation in consignments, it is best to ensure that the place of production is free of pests. Particular attention is needed for consignments from countries where certain B. tabaci-listed viruses, now on the EPPO A1 or A2 quarantine lists, are present. These viruses are also transmitted by biotype B.
Adaptation to risk avoidance at farm level
The biotype B has high pathogenicity and virulence that causes great losses to crops and has consequently resulted in the indiscriminate use of insecticides in attempts to control the pest, in the process also eliminating natural enemies. The direct impacts of whiteflies or through their transmission of viruses can be limited by considering the following control measures.
Monitoring pest populations. Direct observation and use of yellow sticky traps are useful methods for monitoring whiteflies and for early detection and documentation of relative whitefly abundance over time. However, economic injury levels based on these monitoring methods have not been developed.
Natural control. Despite abundant research on natural enemies and non-chemical control of B. tabaci, few natural enemies have been identified and used in biological control. Predators are of the order Coleoptera, such as Delphastus pusillus Gordon, Clitostethus arcuatus (Rossi), Coccinella septempunctata L., Nephaspis oculata Blatvhley, Propylea japonica (Thunberg), P. quatuordecimpunctata L., Coenosia attenuata Stein (Diptera), Crysoperla carnea Stephens (Neuroptera), Orius laevigatus Fieber, O. majusculus Reuter, O. niger Valenciennes, and O. sauteri Poppius. Blennidus peruvianus Dejean (Coleoptera: Carabidae) and Labidura riparia Pallas (Dermaptera: Labiduridae) are soil-inhabiting predators of eggs and nymphs of whitefly. Two diptera, Condylostylus similis Aldrich (Dolichopodidae) and Drapetis sp. Meigen (Empididae), are predators of adult whitefly. Parasitoids are of the genus Encarsia (E. tabacivora Viggiani, E. formosa Gahan, E. lutea Masi, E. cibcensis Lopez Avila, E. nigricephala Dozier, E. reticulata Rivnay, E. deserti Gerling & Rivnay, E. transvesa Timberlake, etc.). Nymphs are affected by entomopathogenic fungi such as Verticillium lecanii Zimmerman, Paecilomyces farinosus Holmsk, and P. fomosoroseus Wize. V. lecanii has been developed as biopesticide. Other entomopathogens are Beuveria bassiana (Balsamo) Vuillemin, and Enthomophthora virulenta Hall and Dunn, which disease develops under high humidity and if sufficient numbers of pathogenic spores are present.
Crop management. Different cultural practices have been studied to reduce immature instars and adult infestation of B. tabaci in sweetpotato. Use of cuttings from healthy fields and only of the end parts of the vines and/or additional disinfection to destroy immature stages is recommended. Maize can be planted as a barrier and/or cucurbits as a trap crop for controlling adults. Further crop rotation or only the cultivation of one host crop per year is recommended as well as the removal of debris and weeds after harvest. Maintaining a minimum distance between crops and use of certified planting material reduces the introduction and spread of whitefly-transmitted viruses in vegetable production. The use of fine mesh protects infestation of whitefly in greenhouses.
Physical control. Yellow attracts whitefly adults. Fixed traps should be installed at planting around fields to capture whitefly adults migrating from other crops. The use of mobile and stationary yellow sticky traps can effectively reduce white fly adult populations.
Chemical control. Avoid use of wide spectrum insecticides. Plant-derived insecticides such as rotenone and rotenone plus pyrethrum reduce nymph population by 60–78%. The chitin inhibitor, buprofezin, has shown high efficacy (>90%) in controlling immature stages. Soaps can also be applied (such as potassium soaps). Chemical control should be based on using action thresholds if three nymphs per leaf are observed.
Further reading
Anderson, P.K., F.J. Morales, A.L. Jones, and R.H. Markham. 2005. Whitefly and whitefly-borne viruses in the tropics: Building a knowledge base for global action. International Center for Tropical Agriculture (CIAT), Cali, Colombia, 351p.
Brown, J.K. 1994. Current status of Bemisia tabaci as a plant pest and virus vector in agroecosystems worldwide. FAO Plant Protection Bulletin 42(1–2): 3–32.
Cisneros, F., and N. Mujica. 1999a. Biological and selective control of the sweetpotato whitefly, Bemisia tabaci (Gennadius) Aleyrodidae. Program report 1997-98. International Potato Center (CIP), Lima, Peru, pp. 255–264. Available online: www.cipotato.org/publications/program_reports/97_98/30whitef.pdf.
Cuellar, M.E, and F.J. Morales. 2006. La Mosca blanca Bemisia tabaci (Gennadius) como plaga y vector de virus en frijol común (Phaseolus vulgaris L.). Revista Colombiana de Entomología 32(1): 1–9.
De Barro, P.J., S.-S. Liu, L.M. Boykin, and A.B. Dinsdale. 2011. Bemisia tabaci: A statement of Species Status. Annual Review of Entomology 56: 1–19.
Della Giustina, W., M. Martinez, and F. Bertaux. 1989. Bemisia tabaci: le nouvel ennemi des cultures sous serres en Europe. Phytoma 406: 48–52.
Gutiérrez-Olivares, M., J.C. Rodríguez-Maciel, C. Llanderal-Cázares, A.P. Terán-Vargas, Á. Lagunes-Tejeda, and O. Díaz-Gómez. 2007. Stability of resistance to Neonicotinoids in Bemisia tabaci (Gennadius) B Biotype, from San Luis Potosí, México. Agrociencia 41: 913–920.
Lourenao, A.L., A.C. Manoel, M.A.C. Miranda, and S.B. Alaves. 2001. Ocurrencia Epizoótica de Verticillium lecanii em Bemisia tabaci Biótipo B (Hemiptera: Aleyrodidae) no Estado do Maranho. Neotropical Entomology 30(1): 183–185.
Mau, R.F.L., and D. Tsuda. 1991. Sweetpotato whitefly Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae).
Morales, F.J., and P.K. Anderson. 2001. The emergence and dissemination of whitefly-transmitted geminiviruses in Latin America. Archives Virology 146: 415–441.
Morales, F., C. Cardona, J. Bueno, and I. Rodríguez. 2006. Manejo de integrado de enfermedades de plantas causadas por virus transmitidos por moscas blancas. Centro Internacional de Agricultura Tropical, DFID, INISAV, Tropical White Fly IPM Project.
OEPP/EPPO. 1990. Specific quarantine requirements. EPPO Technical Documents No. 1008.
Pardina, P.R., A. Luque, C. Nome, E. López, S. Fuentes, and L. Di Feo. 2012. First report of Sweet potato leaf curl virus infecting sweet potato in Argentina. Australasian Plant Disease Notes 7: 157–160.
Perring, T.M. 2001. The Bemisia tabaci species complex. Crop Protection 20: 725–737.
Salas, J., and E. Arnal. 2001. Bemisia tabaci (Gennadius, 1899) biotype B, primer registro para Venezuela utilizando RAPD’s-PCR. Entomotropica 16(3): 191–185.
Sseruwagi, P., J.K. Brown, M.N. Maruthi, J. Colvin, M.E.C. Rey, and J.P. Legg. 2005. Diversity of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) and significance to the epidemiology of whitefly-transmitted viruses in Uganda. IX International Plant Virus Epidemiology Symposium (2005, Lima, Peru). Abstracts. International Potato Center, Lima, Peru, 78 pp.
Sseruwagi, P., J.P. Legg, M.N. Maruthi, J. Colvin, M.E.C. Rey, and J.K. Brown. 2005. Genetic diversity of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) populations and presence of the B biotype and a non-B biotype that can induce silverleaf symptoms in squash in Uganda. Annals of Applied Biology 147: 253–265.
Ueda, S., T. Kitamura., K. Kijima., K.-I. Honda, and K. Kanmiya. 2009. Distribution and molecular characterization of distinct Asian populations of Bemisia tabaci (Hemiptera: Aleyrodidae) in Japan. Journal of Applied Entomology 133: 355–366.
Valverde, R.A., J.G. Sim, and P. Lotrakul. 2004. Whitefly transmission of sweet potato viruses. Virus Research 100: 123–128.