5.1.1 Biocontrol Agents Associated With Potato And Vegetable Pests![]() / Copidosoma koehleri (Blanchard
/ Copidosoma koehleri (Blanchard
1940)
Synonyms: Arrenoclavus koehleri (Blanchard 1940)
Copidosoma uruguayensis (Tachikawa 1968)
Taxonomic position: Insecta, Hymenoptera, Encyrtidae (Encyrtinae)
Authors: V. Cañedo, P. Carhuapoma, E. López, & J. Kroschel
Hosts
Potato tuber moth, Phthorimaea operculella (Zeller) (primary host); Andean potato tuber moth, Symmetrischema tangolias (Gyen); Guatemalan potato tuber moth, Tecia solanivora (Povolny); Tomato leafminer, Tuta absoluta (Meyrick)
Morphology
The ovarian eggs of Copidosoma koehleri are dumbbell-shaped, consisting of the bulb, neck, and enlarged basal portion measuring approximately 0.17 mm in total length. C. koehleri is a polyembrionic species as common in the family Encyrtidae (i.e., two or more); in C. koehleri up to 40 embryos develop from a single fertilized egg. The embryo is divided into two forms: one that will develop into an adult and the other that becomes a soldier responsible for eliminating other species of parasitoids that are in the host.
Larva
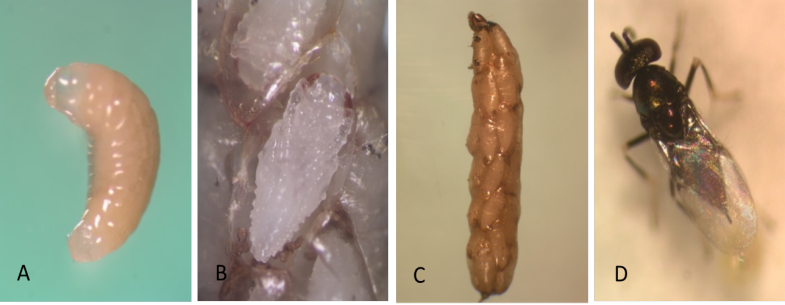
Juvenile L1 larvae are transparent and colorless, but then change to a creamy-white opaque appearance after the ingestion of host tissue (Photo 1A). The potato tuber moth larva spins its silken cocoon as the parasitoids consume its adipose tissue. Parasitized potato tuber moth larvae are unable to pupate because the vital organs are consumed by the internal parasitoids. The parasitized host becomes flaccid and unhealthy in appearance. When the parasitoid larvae devour the contents of the host’s ventricles, their color changes from creamy-white to orange. The host larva is now essentially only a shell surrounding the brood of parasitoids. This host carcass is called a “mummy” (Photo 1B).
Pupa
At the end of larval development, the larva secretes a cocoon sheath to pupate. The pupa is at first entirely white (Photo 1C), but in about 24 hr the eyes become colored with a brick-red pigmentation. Later melanization begins, and the abdominal tergites are the first structures to turn black.
Adult
The small wasps (1.1–1.4 mm, including the ovipositor) have an enlarged pair of middle legs used for jumping. The wings are covered with short hairs. Head and thorax are dark with a metallic green sheen. Antennae from female are long and slender in shape, with a 3-segmented oval clava, whereas the male antenna is always 7-segmented (Photo 1D).
Biology
Parasitism
koehleri is an egg parasitoid of species of the potato tuber moth complex. The female parasitizes moth eggs of all ages but prefers freshly laid eggs. Parasitized host larvae hatch and grow, and inside develop through polyembryony an average of about 35–40 genetically identical C. koehleri wasps from a single-laid C. koehleri egg. Toward the end of host larval development, C. koehleri larvae feed actively on the host tissue and kill the host. Wasp pupation takes place inside the host cuticle (the mummy) from which adult wasps emerge.
Temperature-dependent development
koehleri completes its development from egg to adult at temperatures of 20°–30°C. Total development at 20°C was almost 2.3 times longer (50 days) than at 30°C (22 days). Its total life cycle was not completed at constant temperature of 14°C. The highest mortality of parasitized larvae of P. operculella and mummies were 49% and 52% at 20°C, respectively. Female longevity decreased with high temperatures, from 26 days at 20°C to 12 days at 30°C. The optimum temperature for reproduction ranged from 20° to 25°C. The maximum fecundity of 113 offspring was observed at 20°C. The sex ratio is affected by temperature, with a predominance of males at 30°C (female:male ratio of 1:3.4) and a ratio of 1:1 at 25°C.
The established functions were used to estimate the life-table parameters of C. koehleri and to build an overall stochastic phenology model. Negative r values under 15°C indicate that the population size is decreasing at these temperatures; positive r values between 18°C and 32°C indicate an increase of the population. The finite rate of increase peaked at 22°C (λ=1.0739) and was smallest at 15°C (λ=0.956) and 35°C (λ=0.919), respectively; λ<1 indicates that the population is decreasing. Highest values for the gross reproduction rate and net reproduction rate (Ro) were found at 22°C. The shortest mean generation time (T) was observed at 26°C (48.98 days); the shortest population doubling time (Dt) at 22°C (9.73 days). The optimum temperature for overall population growth ranged from 20°C to 24°C (see Annex 7.4.1).
Economic impact in pest control
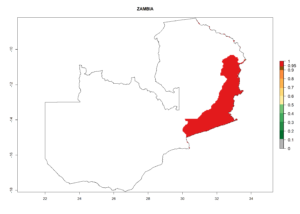
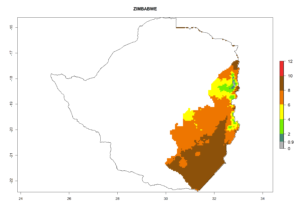
For inundative biological control of the potato tuber moth, C. koehleri was released, in combination with other parasitic wasps like Apanteles subandinus or Orgilus lepidus in several countries (e.g., Australia, South Africa, Zimbabwe, Zambia, and others). C. koehleri complements well with these parasitoids (see sections 5.1.2 and 5.1.3). Classical biological control was very successful in Zimbabwe. After its establishment in Australia, it started to play an important role in P. operculella control after the introduction of a potato integrated pest management program with fewer pesticide applications.
Geographical distribution
Possible region of origin: South America: endemic in Peru, Argentina, Brazil, Uruguay, Chile, Ecuador, and Bolivia
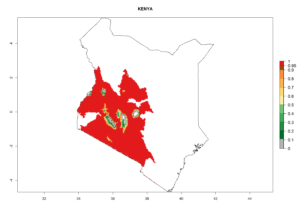
Introduced and established: Australia (including Tasmania), Cyprus, Kenya, India, Mauritius, USA (California), South Africa, St. Helena, Zimbabwe, and Zambia
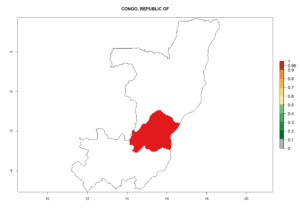
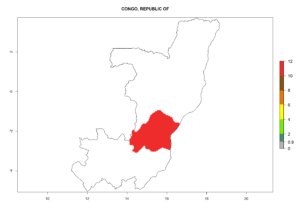
Introduced but establishment not confirmed: Bermuda, Colombia, Greece, Israel, Italy, Japan, Madagascar, New Zealand, Yemen, Seychelles, Tanzania, Venezuela, and DR Congo (Fig. 1).
Potential establishment and efficiency under current and future climates
Changes in global establishment and future distribution
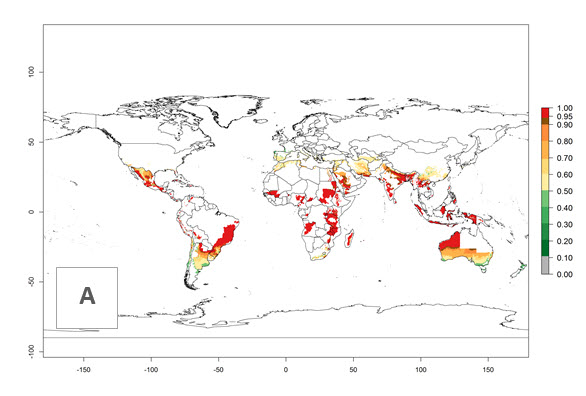
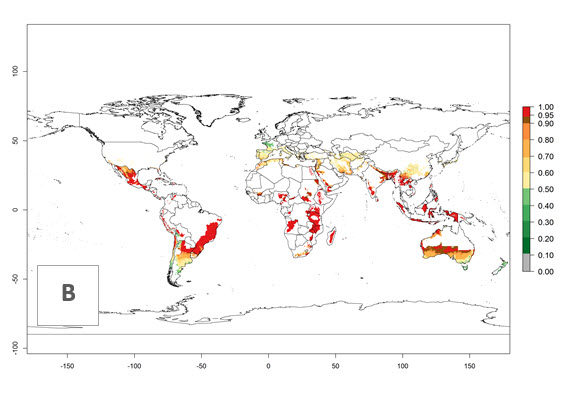
In potato production regions globally, the potato tuber moth, P. operculella, is potentially established at an establishment risk index (ERI)>0.7 (see section 4.1.1). Potential release regions for C. koehleri are those potato production regions where the potato tuber moth has been established, causing significant economic damage in potato fields and stores. Therefore, the establishment index (EI) for the parasitoid C. koehleri is only displayed for those regions where P. operculella can potentially establish. An EI=1 indicates survival of the parasitoid throughout the year—that is, the likelihood of long-term establishment for classical biological control is very high in these regions. However, C. koehleri has also been established in regions with an EI of 0.5–0.6 (indicated by light-cream zones in Peru, Argentina, and Australia in Figure 2; compare with Fig. 1).
| Peru | Argentina | Australia |
Figure 2. EI of Copidosoma koehleri in countries and regions where establishment has been reported according to model predictions of the year 2000. An EI>0.5 (light yellow to red zones) indicates regions with permanent establishment
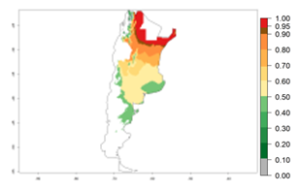
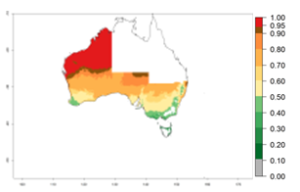
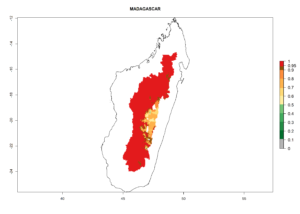
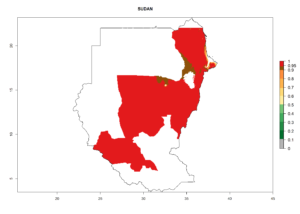
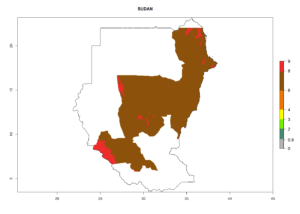
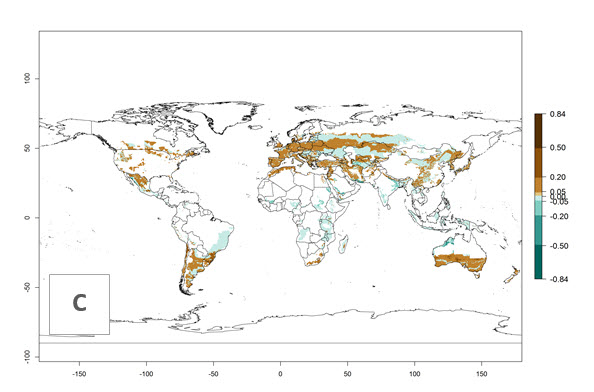
Many regions can be identified where C. koehleri has high EI>0.95 (Fig. 3A). These are in countries and potato-growing regions of South America (Venezuela, Colombia, Ecuador, Peru, Argentina, southeast of Brazil, Bolivia); Central America (Guatemala, Costa Rica, Honduras, Mexico); West and East Africa (Senegal, Cameroon, Sudan, Kenya, Uganda); Asia (India, Indonesia, Nepal, South China); and northwest Australia. Global predictions for 2050 indicate that the establishment of C. koehleri will potentially increase in potato-growing regions of North Africa (Egypt, Morocco, Tunisia); South America (Chile, Argentina, south of Brazil); South Africa; and southern Australia (Fig. 3B). In contrast, a slight reduction in the establishment potential is predicted for some potato-growing regions of Australia, India, and Indonesia, but which will not affect its establishment (Fig. 3C).
 |
 |
 |
|
Figure 3. Establishment and potential distribution worldwide of Copidosoma koehleri, parasitoid of Phthorimaea operculella, according to model predictions, using the EI for the years 2000 (A) and 2050 (B), and changes of the EI between 2000 and 2050 (C), displayed for potato production regions with an ERI>0.7 of its primary host P. operculella. An EI>0.5 indicates regions with potential permanent establishment
Changes in global abundance
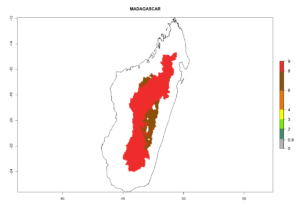
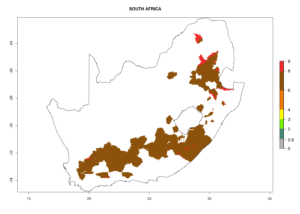
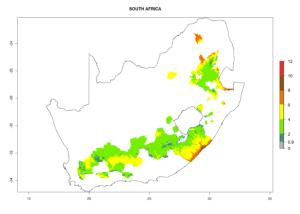
The generation index (GI) reflects the abundance of the population, and it estimates the mean number of generations that may be potentially produced within a given region per year (Fig. 4A, B). The number of generations per year in countries where C. koehleri is established today ranges from 6 to 9. For the year 2050, in some tropical areas of America the GI indicates a potential increase of 1 generation per year (Chile, Argentina, Uruguay, and south of Brazil). In other regions fewer generations will be produced, such as in southeast Brazil, Africa, Asia, and northwest Australia (Fig. 4C).
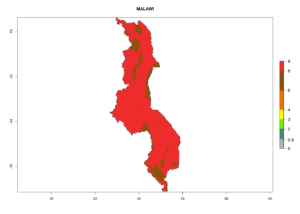
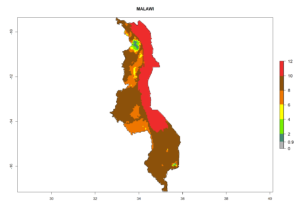
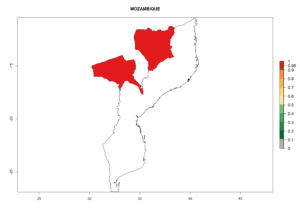
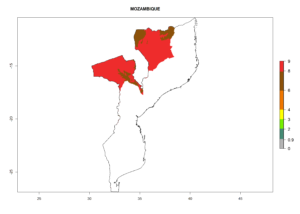
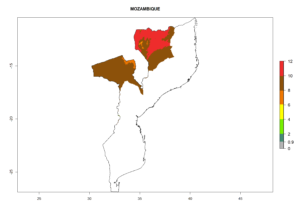
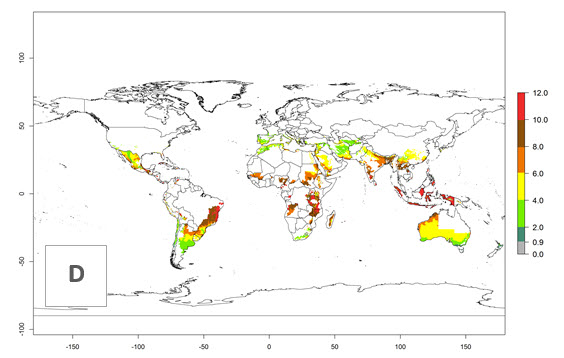
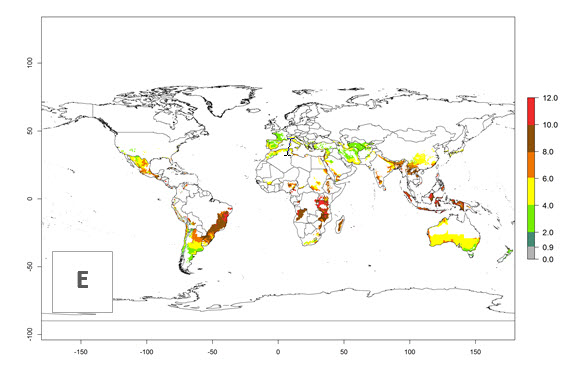
The activity index (AI) highlights not only the establishment but also the potential spread and efficiency of C. koehleri to control its host. The AI for the year 2000 reveals an activity of C. koehleri of 4–6 in countries where the species is present today. For potato production regions of Zimbabwe, where the parasitoid was very successful in biological control, an AI of up to 10 was estimated (Fig. 4D, compare with Fig. 1). Predictions of change for the 2050 climate change scenario show a slight increase in the potential growth by a factor of 1–3 in some potato-growing areas of Peru, Bolivia, Chile, and southeast Brazil in South America; in countries of southeast Africa (e.g., Mozambique), and south of Australia. In contrast, a reduction of the potential growth will be expected in Indonesia (Fig. 4E, F).
| GI | AI | |
| 2000 | 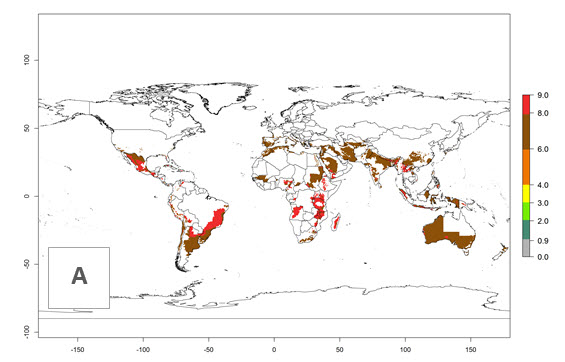 |
 |
| 2050 | 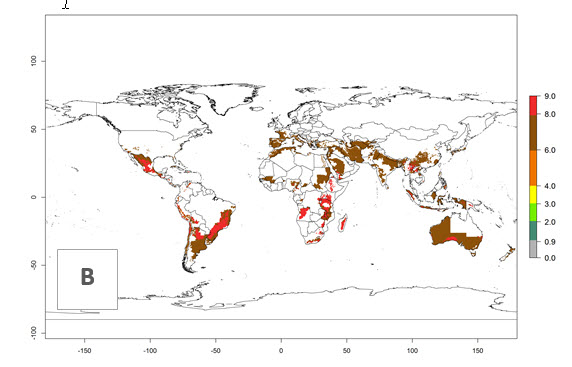 |
 |
| Index change (2000 – 2050) | 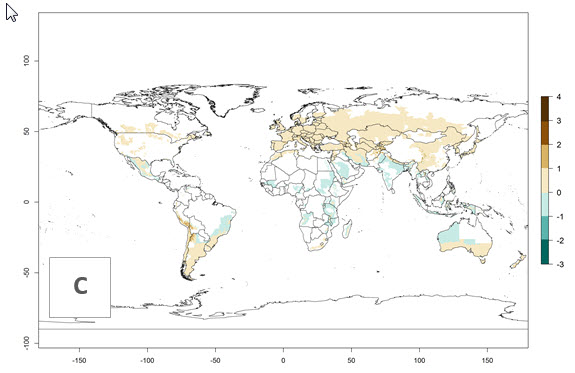 |
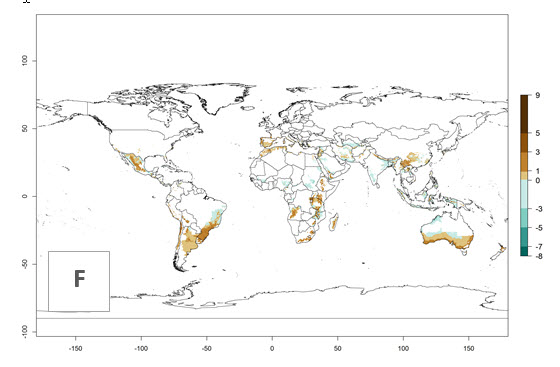 |
Figure 4. Changes in abundance (GI, number of generations/year) and activity (AI, potential population growth) of Copidosoma koehleri in potato production regions worldwide according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F), displayed in potato production regions with an ERI>0.7 of its primary host Phthorimaea operculella
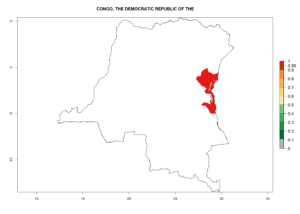
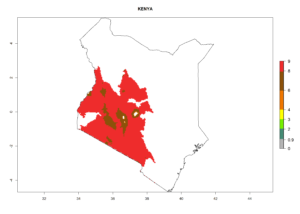
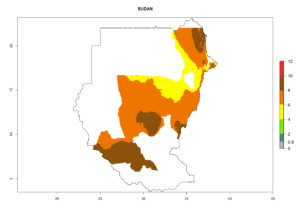
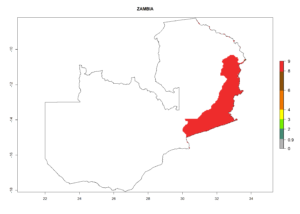
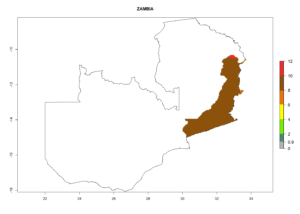
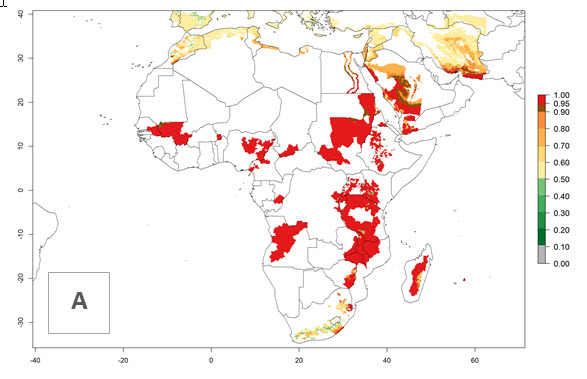
Changes in regional establishment and distribution in Africa
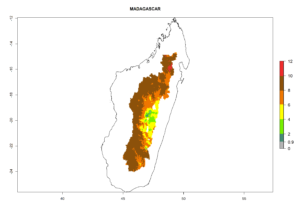
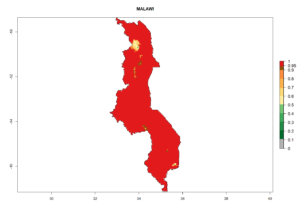
In Africa, C. koehleri has been introduced and established in Kenya, South Africa, Zimbabwe, and Zambia. It has also been introduced into Madagascar, the Seychelles, Yemen, Tanzania, and DR Congo; but establishment was not further validated and confirmed. According to the mapping results, however, it could have been expected to establish successfully in all these countries (Fig. 5A).
 |
 |
 |
|
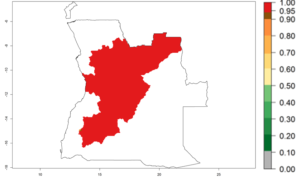
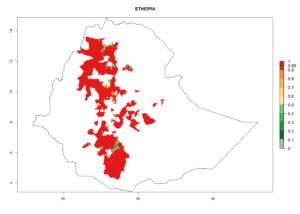
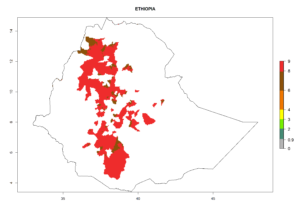
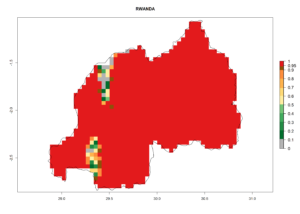
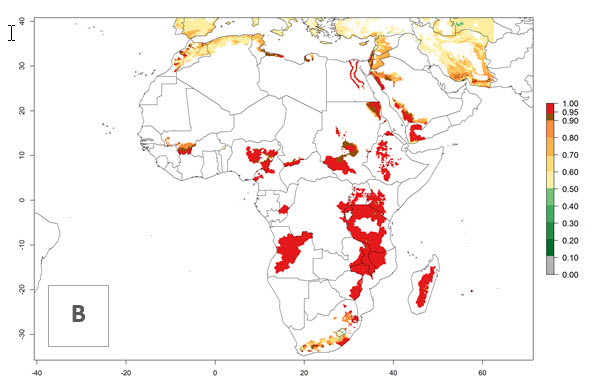
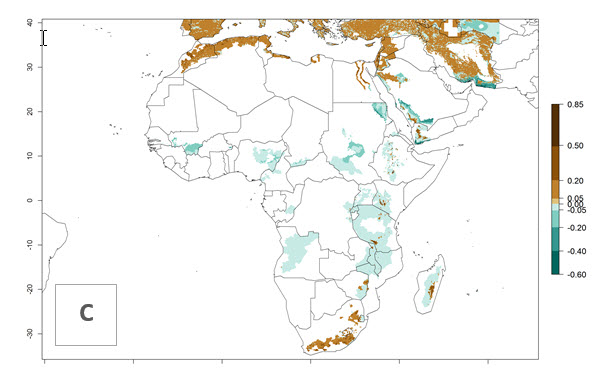
Figure 5. Establishment and potential distribution of Copidosoma koehleri in Africa according to model predictions, using the EI for the years 2000 (A) and 2050 (B), and changes of the EI between 2000 and 2050 (C), displayed for potato production regions with an ERI>0.7 of its primary host . An EI>0.5 indicates regions with potential permanent establishment.
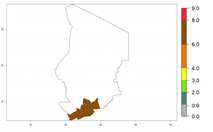
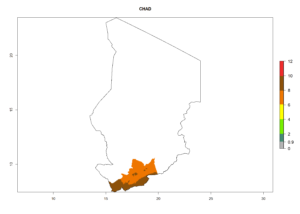
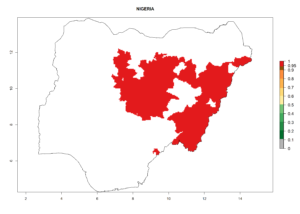
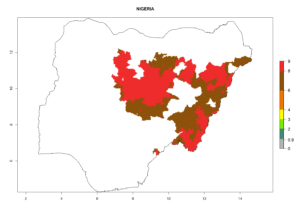
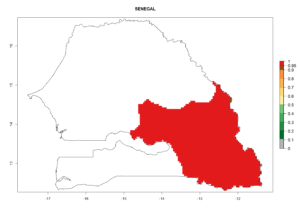
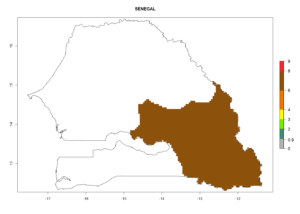
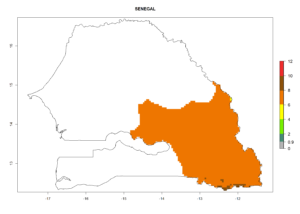
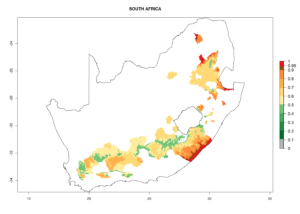
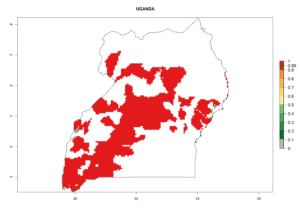
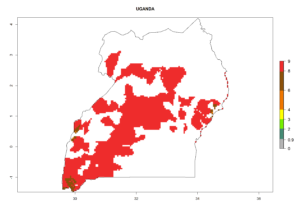
In tropical eastern Africa, an EI>0.95 indicates that C. koehleri could potentially establish according to the prevalent temperature conditions in the potato-growing regions of Angola, Ethiopia, Chad, Congo, Kenya, Madagascar, Malawi, Mali, Mozambique, Nigeria, Rwanda, Senegal, Sudan, Uganda, Zambia, Zimbabwe, and Tanzania (Fig. 5A). Climate change is expected to increase the establishment potential in South Africa and countries of North Africa (Fig. 5B, C).
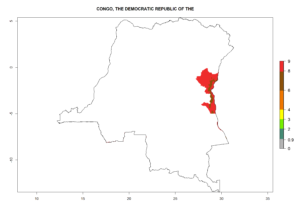
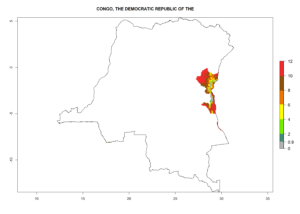
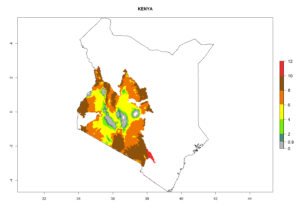
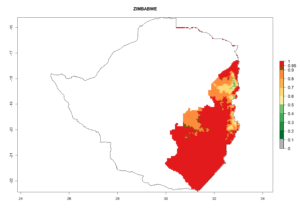
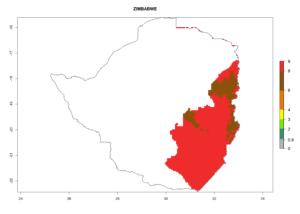
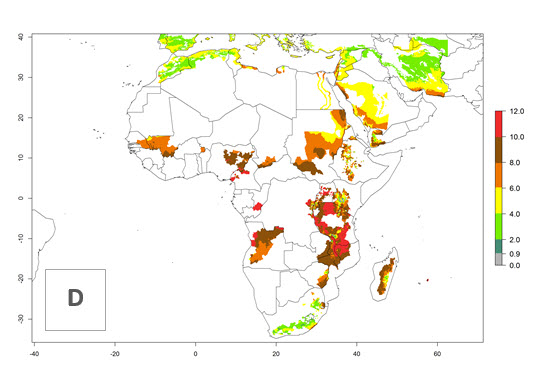
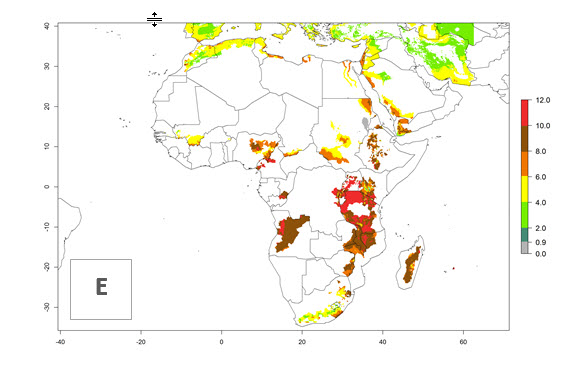
Changes in regional abundance in Africa
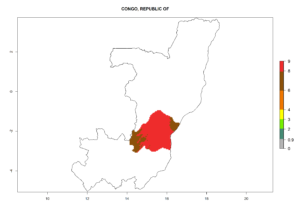
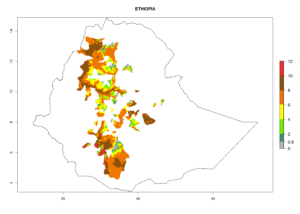
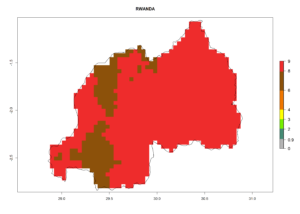
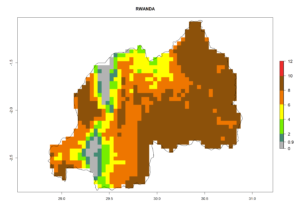
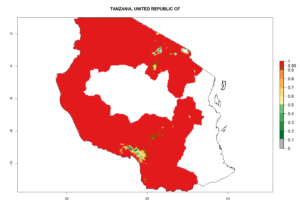
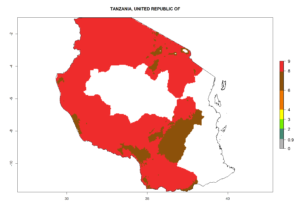
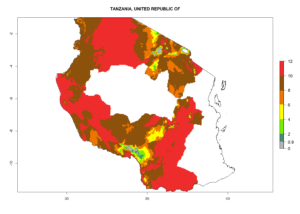
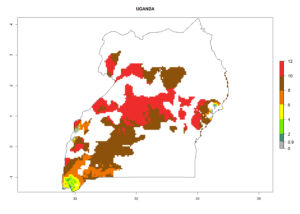
In many potato-growing areas of Africa, the GI reveals 6–9 generations per year (Fig. 6A). Predictions for the year 2050 indicate an increase in generation numbers per year in potato highland production regions of East Africa, South Africa, and some parts of Madagascar. In contrast, the number of generations could potentially reduce in countries of the Sahel such as Senegal and Sudan (Fig. 6B, C). The activity and spread potential of the parasitoid is expected to increase in potato-growing areas of East and South Africa. Especially for Angola, Ethiopia, Kenya, Madagascar, Malawi, South Africa, Tanzania, and Uganda, a high C. koehleri activity of AI>8 is projected (Fig. 6D–F).
| GI | AI | |
| 2000 | 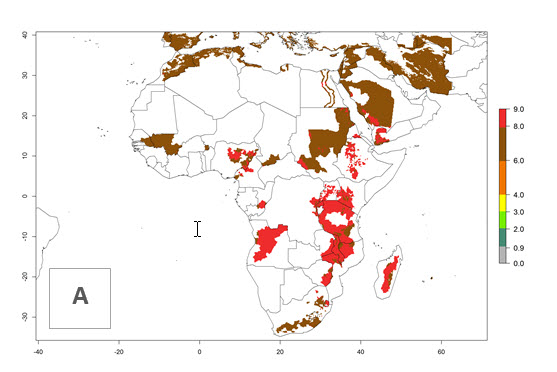 |
 |
| 2050 | 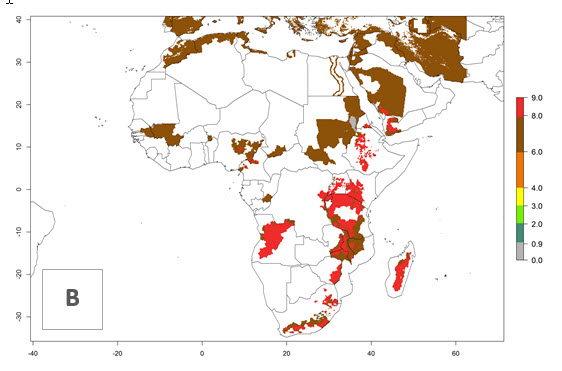 |
 |
| Index change (2000 – 2050) | 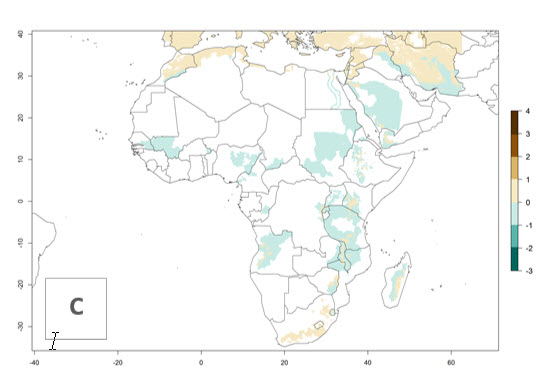 |
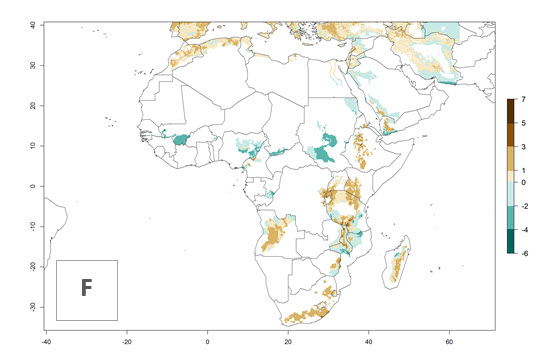 |
Figure 6. Changes in abundance (GI, number of generations/year) and activity (AI, potential population growth) of Copidosoma koehleri in Africa according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F), displayed in potato production regions with an ERI>0.7 of its primary host Phthorimaea operculella.
Potential release areas in Africa
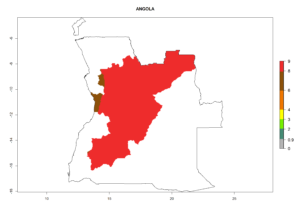
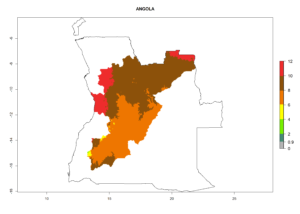
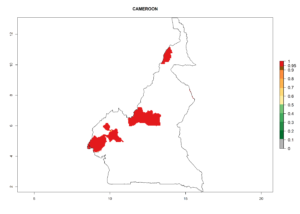
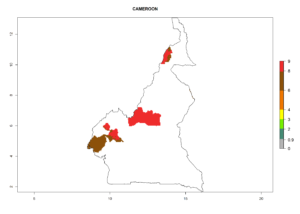
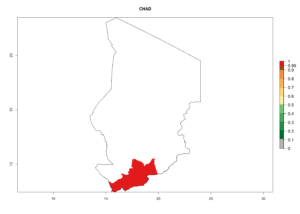
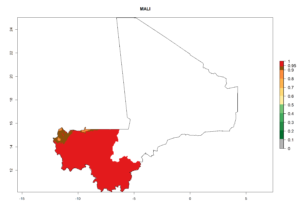
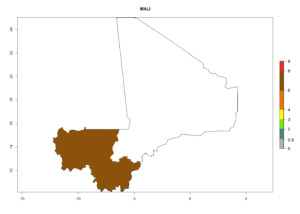
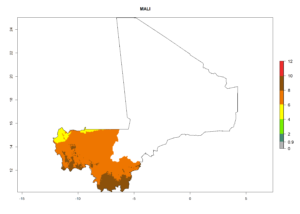
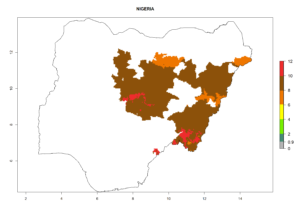
Considering an ERI>0.7 for P. operculella (section 4.1.1) in potato-growing areas of Africa, the potential countries to release C. koehleri under the current climate are Angola, Cameroon, Chad, Congo, DR Congo, Ethiopia, Kenya, Madagascar, Malawi, Mali, Mozambique, Nigeria, Rwanda, Senegal, South Africa, Sudan, Tanzania, Uganda, Zambia, and Zimbabwe (Fig. 7). C. koehleri has been shown to establish at an EI>0.5; in all suggested countries the likelihood of establishment is expected to be higher in almost all potato-growing regions with an EI>0.95. This high establishment potential is associated with a GI>8 (i.e., more than 8 generations per year) and an AI>8.
Figure 7. EI, abundance (GI, number of generations/year), and activity (AI, potential population growth) of Copidosoma koehleri in selected African countries according to model predictions for the year 2000, displayed in potato production regions with an ERI>0.7 of its primary host Phthorimaea operculella. An EI>0.5 indicates regions with permanent establishment.
Risks to no-targets
No risks are reported. The three potato tuber moth species P. operculella, S. tangolias, and T. solanivora are the only known hosts of C. koehleri. In regions where P. operculella has been accidently introduced, generally local parasitoids specific to P. operculella are absent. In such situations, local parasites comprise oligo- or polyphagous species in general. C. koehleri might dominate local species because of its high specificity to potato tuber moth species.
Further reading
Callan, E.M. 1974. Changing status of the parasites of potato tuber moth Phthorimaea operculella (Lepidoptera: Gelechiidae) in Australia. Entomophaga 19: 97–101.
Cruickshank, S., and F. Ahmed. 1973. Biological control of potato tuber moth Phthorimaea operculella (Zell.) (Lep.:Gelechiidae) in Zambia. Technical Bulletin of the Commonwealth Institute of Biological Control: 147–162.
Doutt, R.L. 1947. Polyembryony in Copidosoma koehleri Blanchard. The American Naturalist 81: 435–453.
Horne, P., and J. Page. 2008. Integrated Pest Management dealing with potato tuber moth and all other pests in Australian potato crops. In Integrated Pest Management for the Potato Tuber Moth, Phthorimaea operculella Zeller – a Potato Pest of Global Importance. J. Kroschel and L. Lacey eds., 111–117. Tropical Agriculture 20, Advances in Crop Research 10. Weikersheim, Germany: Margraf Publishers.
Kfir, R. 1981. Fertility of the polyembryonic parasite Copidosoma koehleri, effect of humidities on life length and relative abundance as compared with that of Apanteles subandinus in potato tuber moth. Annals of Applied Biology 99: 225–230.
Kfir, R. 1989. Effect of pesticides on Copidosoma koehleri Blanchard (Hymenoptera: Encyrtidae), a parasite introduced into South Africa for biological control of the potato tuber moth. Journal of the Entomological Society of Southern Africa 52: 180–181.
López, C.E. 2006. Influencia de la temperatura en el ciclo biologico de Copidosoma koehleri Blanchard parasitoide de Phthorimaea operculella (Zeller). BSc thesis, Universidad Nacional Federico Villarreal, Lima, Peru.
Mitchell, B.L. 1978. The biological control of potato tuber moth Phthorimaea operculella (Zeller) in Rhodesia. Rhodesia Agricultural Journal 75: 55–58.
Pokharkar, D.S., and R.R. Jogi. 2000. Biological suppression of potato tubermoth, Phthorimaea operculella (Zeller) with exotic parasitoids and microbial agents under field and storage conditions. Journal of Biological Control 14: 23–28.
Pucci, C., A.F. Spanedda, and E. Minutoli. 2003. Field study of parasitism caused by endemic parasitoids and by the exotic parasitoid Copidosoma koehleri on Phthorimaea operculella in Central Italy. Bulletin of Insectology 56: 221–224.
Segoli, M., A. Bouskila, A.R. Harari, and T. Keasar. 2009a. Developmental patterns in the polyembryonic parasitoid wasp Copidosoma koehleri. Arthropod Structure and Development 38: 84–90.
Syndikus, K. 2010. Extent of losses and possibilities to optimize pest management in potato cropping systems in Kenya. MSc thesis, University of Hohenheim, Stuttgart, Germany.
Whiteside, E.F. 1980. Biological control of the potato tuber moth (Phthorimaea operculella) in South Africa by two introduced parasites (Copidosoma koehleri and Apanteles subandinus). Journal of the Entomological Society of Southern Africa 43: 239–255.