4.3.4 Vegetable Pests / Greenhouse whitefly, Trialeurodes vaporariorum (Westwood 1956)![]()
Synonym: Aleyrodes vaporariorum (Westwood1856)
Taxonomic position: Insecta: Hemiptera, Aleyrodidae
Authors: H. Gamarra, P. Carhuapoma, N. Mujica, J. Kreuze, & J. Kroschel
Common names
South American glasshouse or greenhouse whitefly (English), weisse Fliege (German), Aleurode des serres (French), Aleirode delle serre (Italian), Witte vlieg (Dutch)
Hosts
Cassava (Manihot esculenta Crantz), cotton (Gossipum barbadense L.), bean (Phaseolus vulgaris L.), huacatay (Tagetes minuta L.), tomato (Solanum lycopersicum L.), lantana (Lantana camara L.), potato (Solanum tuberosum L.), sunflower (Helianthus annus L.), faba bean (Vicia faba L.), and a large host range of ornamental plants, including species of geranium (Pelargonium graveolens L’Her.), poinsettias (Euphorbia pulcherrima Willd. ex Klotzsch) Fuchsia spp., Gerbera spp., Pelargonium spp., Solanum spp., Chrysanthemun spp., and Primula spp.
Detection and identification
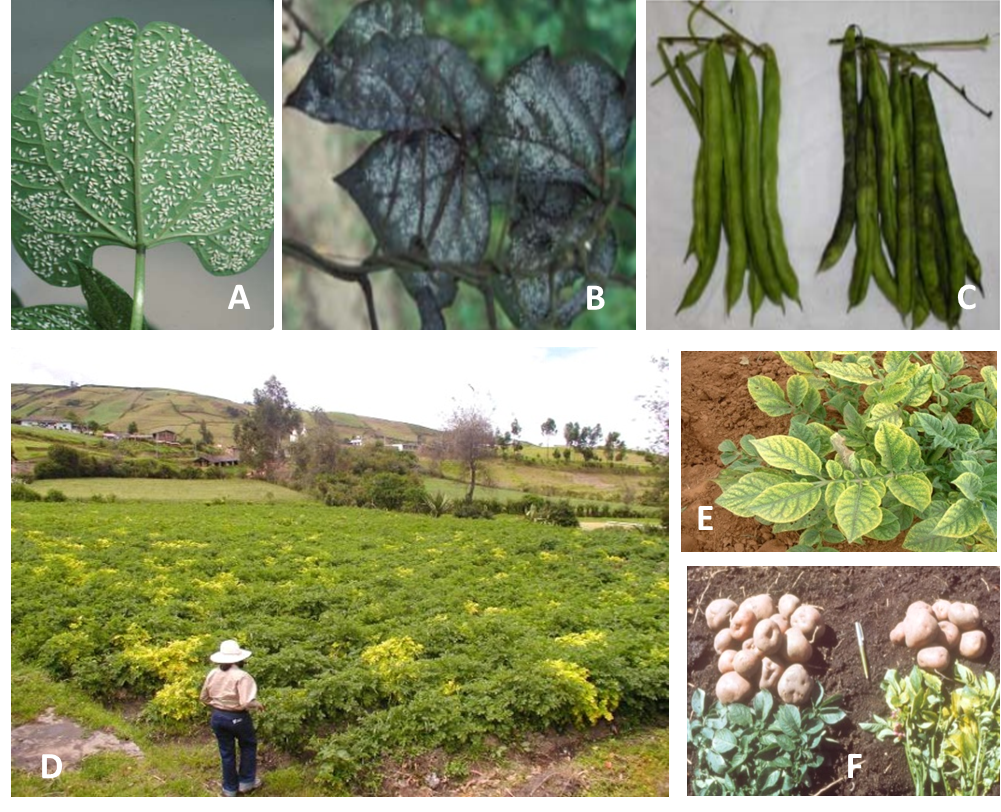
Adults and nymphs of Trialeurodes vaporariorum cause three types of damage to plants: direct suction of plant sap, honeydew covering plants caused by extensive sugar secretions, and transmission of plant viruses. Damage by sucking sap from plant tissue can be serious when whitefly populations are high (e.g., tomato plants turn yellow, look mottled, and have stunted growth causing wilting and defoliation and thereby seriously reducing crop yield). Heavy feeding by whitefly can eventually kill plants. Sugary excretions and honeydew interfere with normal photosynthetic processes and support the growth of fungi. This leads to sooty mold, which in some cases can affect the quality of the harvested product (Photo 1A, B). Most harmful is the ability of adults to transmit plant viruses, mainly from the genus Crinivirus (family Closteroviridae) such as Potato yellow vein virus (Photo 1C). Symptoms are clearing of secondary and tertiary leaf midribs, followed by vein yellowing with green interveinal spaces. Tomato infectious chlorosis virus (TICV) is also transmitted by T. vaporariorum, and initial symptoms appear as interveinal yellowing with green veins mostly on mature lower leaves, while the rest of the plant tends to appear “normal.” As the disease progresses, similar symptoms can be observed on young upper leaves. Subsequently, the symptomatic leaves become pale-white, necrotic, dry and curled. Infected leaves (especially older ones) may also turn red. Early infection can also affect the fruit set, color, and quality, resulting in substantial yield loss. TICV causes symptoms, termed “yellow leaf disorder,” showing interveinal chlorosis, leaf brittleness, and limited necrotic flecking or leaf bronzing. Although symptoms of the Beet pseudo yellow virus are similar to those of TICV, this virus also induces stunting, poor plant growth, and interveinal yellowing or reddening.
Morphology
Egg
When first laid, eggs (0.23 x 0.1 mm) are transparent to pale cream-yellow, which turn to brown-black (Photo 2A).
Nymphal instars
First nymphal instars (0.23 x 0.11 mm), called “crawlers,” are near-transparent, flat, and oval (Photo 2B). Second nymphal instars (0.38 x 0.23 mm) are translucent and oval with wavy edges (Photo 2C). Third nymphal instars are larger in size (0.54 x 0.33 mm) but morphologically similar to the second nymphal instar (Photo 2D).
Pupa (fourth nymphal instar)
Fourth nymphal instar (0.73 x 0.45 mm) is oval, flat, and almost transparent (Photo 2E). As development progresses it becomes opaque and is called pupa. The pupa is elliptical with characteristic long erect threads of wax. Profile appears to project out relative to the surface of the sheet; in more developed pupae, close to adult emergence, the eyes are clearly visible.
Adult
Wings of newly emerged adults (0.75 x 1.10 mm) are clear but in time become covered with a white wax. The female is larger than the male (Photo 2F).

Biology
Greenhouse whiteflies lay eggs fixed to the leaf with the help of a peduncle. On hairy leaves the eggs are laid randomly across the leaf surface. First nymphal instar has tiny legs that allow it to move across the leaf surface. After hatching, it may travel a short distance until it successfully probes the leaf to obtain sap, where it then settles to feed. It remains at this location until it has developed into an adult. Before molting into the next nymphal instar, the body swells and is more rounded. Adults can fly within a few hours and begin feeding by piercing leaves and sucking plant sap, which they continue to do for the rest of their lives. During the first few days, adults move from old leaves to younger leaves on the same or different plant. Thereafter they spend most of their time on the undersides of the topmost leaves, but will fly when disturbed. The female lives 5–8 days and is differentiated from the male by her size. It feeds and lays its eggs on the abaxial surface of leaves. Adults mate as soon as they emerge, but there may be a period of pre-oviposition of 1 or 2 days, depending on the temperature. T. vaporariorum can reproduce parthenogenetically, giving rise to progeny of exclusively males.
Temperature-dependent development
T. vaporariorum completed its development from egg to adult at all temperatures of 15°–28°C but not at 10º–32°C (see Annex 7.3.12). Whitefly total development was almost twice as long at 15ºC (46.71 days) than at 28ºC (21.87 days). Estimated lower thresholds for immature stages were 9.45ºC (egg), 6.26ºC (nymph), and 10.64ºC (pupa). For all three immature stages, lowest mortality occurred at 25ºC (1%) and the highest at 28ºC (30.23%). The pupa was more vulnerable to unfavorable temperatures compared with other stages. Optimal temperature for survival was 20°–25ºC (93–96% survival of the entire immature stages). Significant differences were observed in the longevity between males and females. Females lived twice as long as males at temperatures >15ºC. The lowest senescence rate was observed at 18ºC. Highest fecundity was found at around 20ºC (42.3 eggs/female).
The established functions (see Annex 7.3.12) were used to estimate the life-table parameters of T. vaporariorum and build an overall deterministic phenology model. Predicted life-table parameters at constant temperatures show that T. vaporariorum populations develop within the temperature range of 16°–27°C. The intrinsic rate of natural increase (rm) augments almost linearly with increasing temperature to reach a maximum at 23ºC (0.039), which then decreased at 24ºC (0.036). The negative r values below 16ºC and above 27ºC indicate that population size is decreasing due to high mortality and limited reproduction. Similarly, finite rate of increase peaked at 23ºC (λ = 1.040) and was smallest when exposed to <16ºC and >27ºC. The highest values for both reproductive parameters (gross reproductive rate and net reproductive rate, Ro) were found between 20°C and 22ºC. The shortest mean generation time (T) and doubling time (Dt) were observed at 26ºC (24.9 days) and 23ºC (17.5 days), respectively. The optimum temperature for overall population growth ranged 20°–24ºC.
Deterministic simulation of life-table parameters under prevailing temperature conditions of two agro-ecological zones in Peru showed that lowland (La Molina: 12º 05′ S, 76º 57′ W, 250 masl, with a mean annual precipitation of 6.4 mm and mean temperature of 19.7ºC) presents better temperature conditions than highland (Cusco: Anta: 13º 25′ S, 72º 18′ W, 3392 masl, with a mean annual precipitation of 760 mm and mean temperature of 15.5ºC) for greenhouse whitefly population increase in all evaluated seasons. According to the mean generation time (T), we predicted 11 and 7.4 generations per year in lowland and highland agro-ecologies, respectively. Additionally, for the different seasons, 2.1 (mean temperature of 16.3ºC), 2.9 (mean temperature of 18.8ºC), 2.3 (mean temperature of 19ºC), and 3.2 (mean temperature of 23.6ºC) generations are expected for winter, autumn, spring, and summer, respectively, under lowland conditions.
Means of movement and dispersal
Adults are active during the day, starting in the early morning hours. After adults emerge, initial flights are very short; but after several days adults move greater distances of up to 2 m/day. Dispersal from field to field occurs with winds. Infested plants and planting material are responsible for long-distance movements between regions, countries, and continents.
Economic impact
The whitefly T. vaporariorum is one of the most important pests worldwide due to the large number of virus diseases it transmits; its wide range of cultivated and wild hosts; and its wide geographical distribution in tropical, subtropical, and temperate regions. In beans, T. vaporariorum can cause losses of up to 50%. Potato yellow vein virus reduces tuber number and size, causing losses of 28–50%. TICV causes annual losses to tomato growers in California of almost US $2 million. Tomato chlorosis virus is a severe disease in the southern France affecting tomato yields by some 5–30%. Beet pseudo yellow virus causes extensive damage in crops of lettuce grown under cover in France and the Netherlands. Strawberry pallidoses associated virus reduced runner production and root growth by 15–20% in strawberry greenhouse-grown plants. However, its major impact in yield loss is in mixed infections with other strawberry viruses.
Geographical distribution (permanent establishment)
T. vaporariorum originated in tropical or subtropical America, probably Brazil or Mexico. Currently it has become a global pest with a distribution reported in more than 50 countries (Fig. 1). T. vaporariorum is a widely distributed pest of ornamental and horticultural plants. It is not native to Europe but has become successfully established in glasshouses. T. vaporariorum also occurs under field conditions in North America.
| Africa | Algeria, Ethiopia, Kenya, Ghana, Madeira, Morocco, Reunion, South Africa, Tunisia, Zimbabwe |
| Asia | Bangladesh, China (Begin, Jilin, Ningxia, Guizhou, Qinghai, Hong Kong), India, Indonesia (Agam, Tanah Datar), Iran, Israel, Japan, Jordan, Republic of Korea, North Korea, Singapore, Sri Lanka, Philippines, Turkey, Uzbekistan |
| Europe | Albania (Saranda, Gjirokastra), Austria, Azores, Belgium, Bosnia-Herzegovina, Bulgaria, Canary Islands, Croatia, Czech Republic, Denmark, Estonia, Finland, France (northern region), Greece, Germany (northern region), Hungary (Budapest), Iran, Ireland, Italy, Latvia, Lithuania, Madeira Island, Malta (Zejtun, Birkirkara, Gnien il-Kbir, Zebbiegh, Silema), Montenegro, the Netherlands, Norway, Poland (Masurian Lakeland, Wielkopolska-Kujawy Lowland, Mazovian Lowland, Bialowieża Primeval Forest, Kraków-Wieluń Upland, Lublin Upland, OrawaNowy Targ Basin), Portugal, Madeira, Portugal (western region), Romania, Russian Federation, Russian Far East, Serbia, Spain (Almeria, Catalonia), Sweden, Switzerland, United Kingdom, Channel Islands, Yugoslavia (former), Turkmenistan |
| North America | Bermuda, Canada, USA (Arizona, Hawaiian islands of Honolulu, Kauai, Oahu, Molokai, Lanai, Maui, and Hawaii, Southern California) |
| Central America and the Caribbean | Barbados, Belize, Costa Rica, Cuba, Dominican Republic, El Salvador, French Guiana, Guadeloupe, Guatemala, Honduras, Jamaica, Martinique, Mexico, Netherlands Antilles, Panama, Puerto Rico |
| South America | Argentina (Cordova), Bolivia, Brazil, Chile (Valparaiso), Colombia, Ecuador, Peru, Uruguay, Venezuela |
| Oceania | Australia, New Zealand, Papua New Guinea |
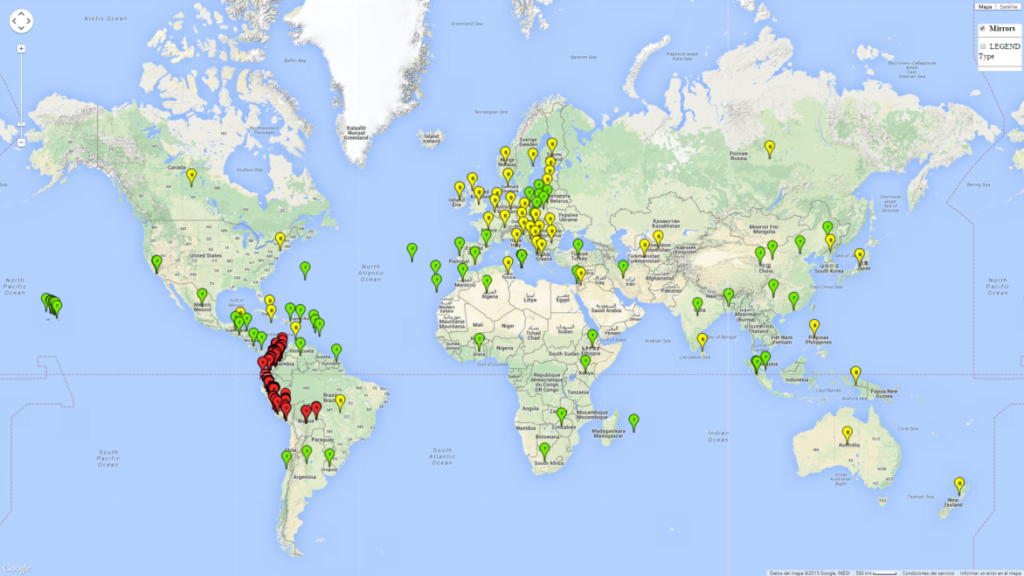
Phytosanitary risks
The greenhouse whitefly is present almost throughout the globe, and is therefore not generally considered as a quarantine pest. However, owing to risks of severe pest outbreaks in Europe and Japan, it is listed by the Food and Agriculture Organization as a quarantine pest. Risks exist primarily to the glasshouse industry in countries of northern Asia. T. vaporariorum in particular is difficult to control because of its polyphagous nature and resistance to many insecticides. Countries that already host many whitefly-transmitted viruses in indigenous plants and weeds associated with local populations of T. vaporariorum with narrow host ranges may be at risk of invasion of T. vaporariorum biotypes with broader host ranges. This situation could lead to virus transmission to susceptible cultivated plants.
Risks mapping under current and future climates
Global Risks
Changes in establishment and future distribution
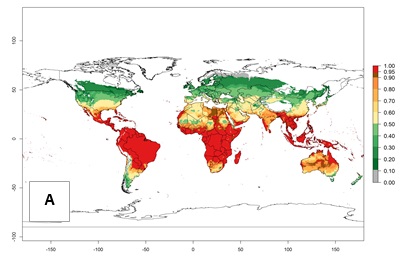
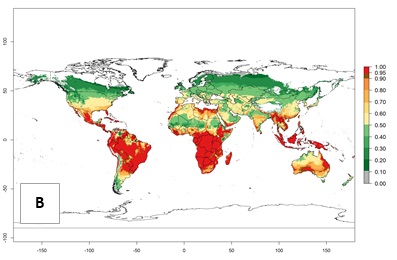
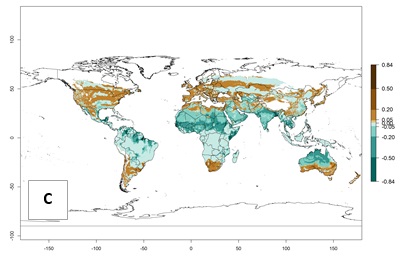
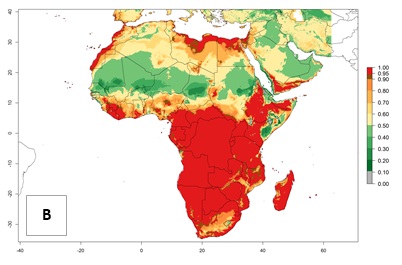
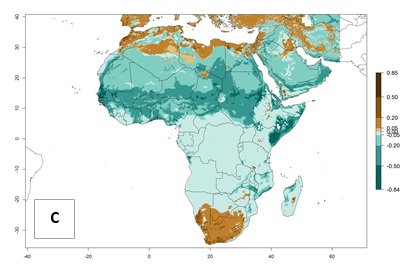
Regions with an establishment risk index (ERI)>0.95 indicate temperature conditions at which a certain proportion of T. vaporariorum immature stages are expected to survive throughout the year (Fig. 2A). The highest likelihood of establishment is in tropical and subtropical areas of the Southern Hemisphere of Africa, Asia, Oceania, and South and Central America (Fig. 2A). Here, the pest is currently widely distributed and established in the field (Fig. 1). In zones where the ERI drops below the maximum number of 1, the likelihood of long-term establishment is reduced, but T. vaporariorum also occurs in regions with an ERI>0.6–0.8 as in southern regions of China, South Africa, central Argentina (Cordoba), and some areas of southern European countries (Greece, Portugal, Spain, Italy). In contrast, T. vaporariorum is least established in Europe and North America (Canada), where it has become successfully established only in glasshouses (ERI<0.6). In the year 2050, risks of T. vaporariorum establishment is predicted to lessen in current high-risk areas (ERI>0.8) of tropical and subtropical regions globally due to high temperatures. Instead, the temperate regions of Asia, North America, and Europe will become more suitable but still remain with a very low risk of establishment (ERI<0.6) (Fig. 2B).
Figure 2. Changes in establishment and potential distribution of the greenhouse whitefly, Trialeurodes vaporariorum, worldwide according to model predictions, using the ERI for the years 2000 (A) and 2050 (B), and changes of the ERI between 2000 and 2050 (C). An ERI>0.6 is associated with potential permanent establishment.
Changes in abundance
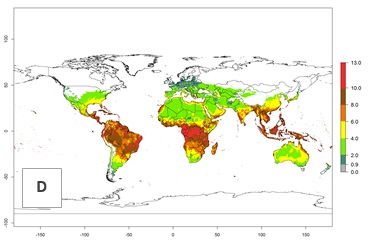
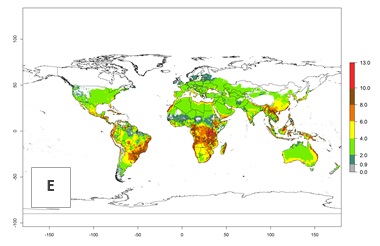
The generation index (GI) change indicates that in most tropical regions, an increase of 1–2 generations is forecast, including northern, southern, and eastern South America; Southern Africa; the Caribbean; Asia (China, Iran, Nepal, Afghanistan, Bhutan, Indonesia); and Oceania (Australia, New Zealand, Papua New Guinea) (Fig. 3C). Globally, simulated GIs gave reasonable predictions when compared with generation numbers reported in the literature (e.g., under tropical conditions in Colombia (mean temperature of 25°C; a mean number of 11 generations per year has been reported). Estimations made by the GI are consistent with these data. Global maps of the activity index (AI) of T. vaporariorum for the year 2000 estimate a factor of population growth potential of 6–13 in tropical regions of South America, the Caribbean, Africa, Asia, and Oceania (Fig. 3D). Predictions of changes for the 2050 climate change scenario indicate a small increase in the potential growth of T. vaporariorum in temperate regions such as North America and Europe (Fig. 3E, F). Instead, increasing temperatures along the equator will potentially reduce T. vaporariorum activity.
| GI | AI | |
| 2000 | 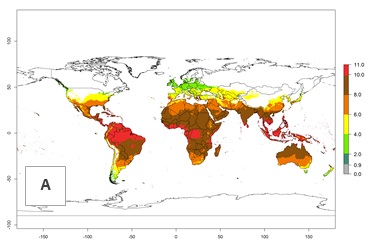 |
 |
| 2050 | 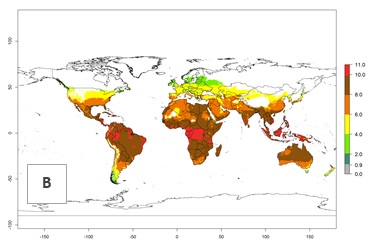 |
 |
| Index change (2000 – 2050) | 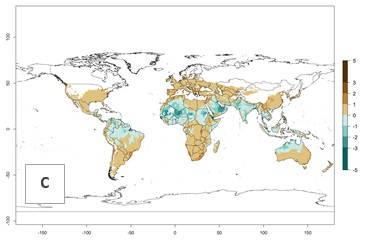 |
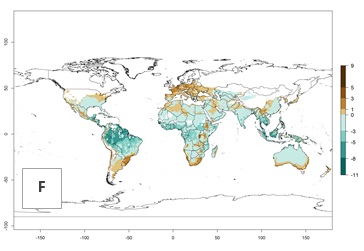 |
Figure 3. Changes in abundance (GI, damage potential) and activity (AI, potential population growth) of the greenhouse whitefly, Trialeurodes vaporariorum, worldwide according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
Regional Risks for Africa
Changes in establishment and future distribution
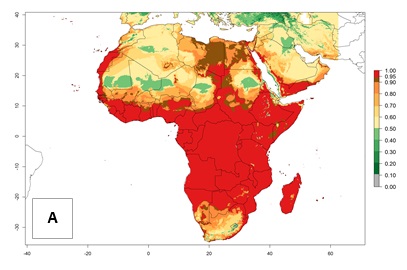
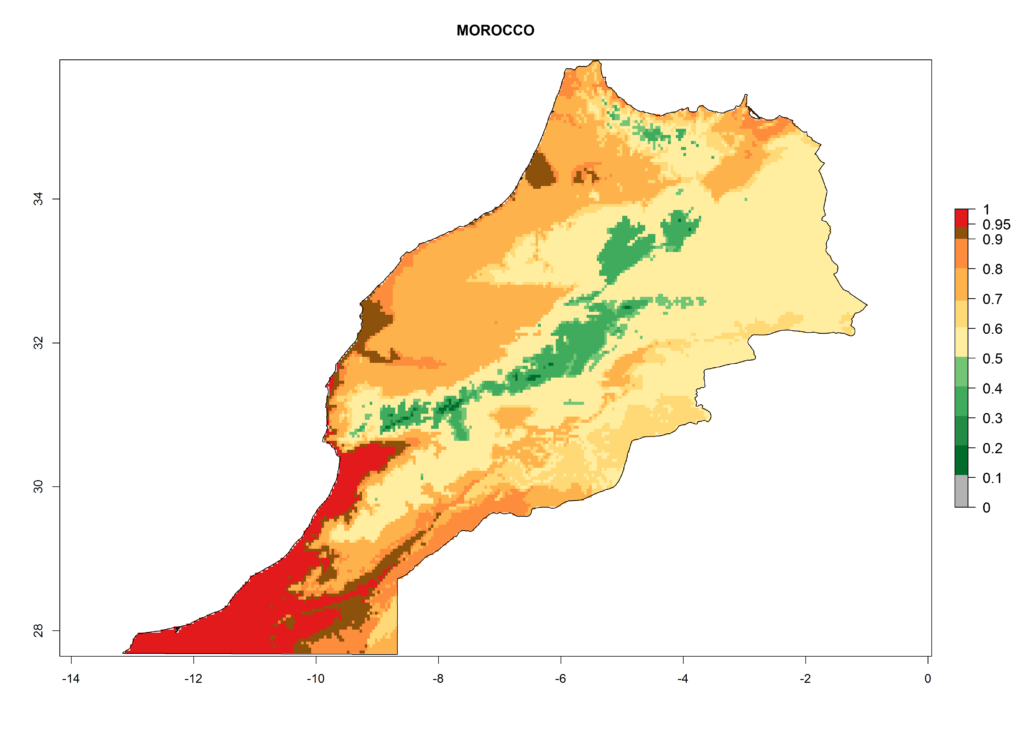
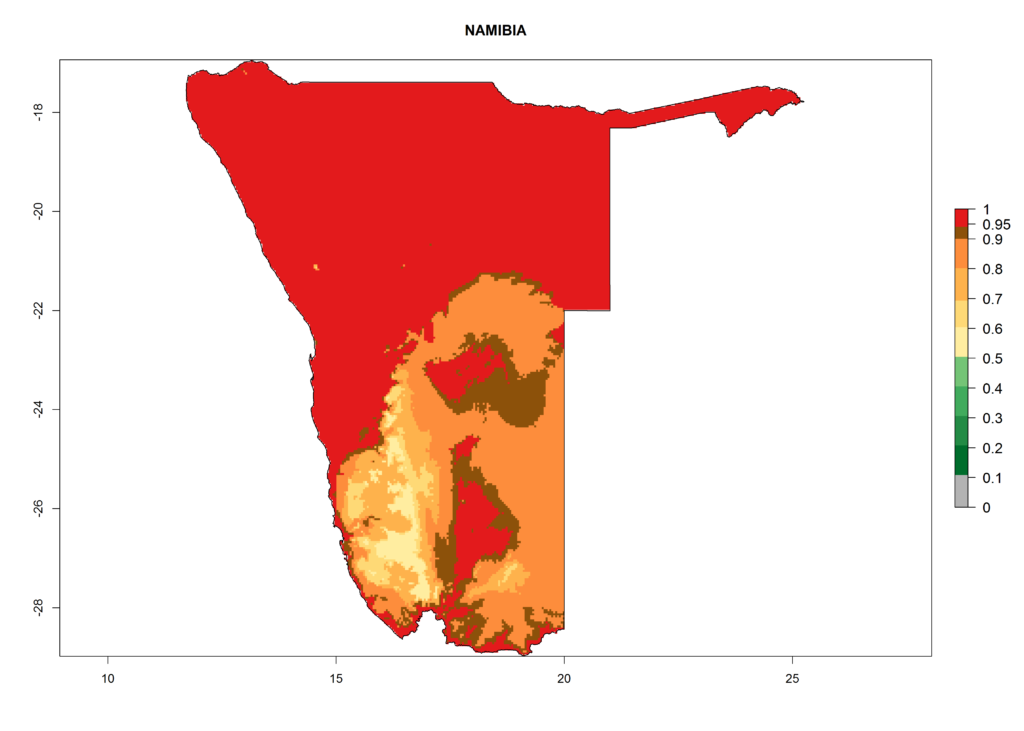
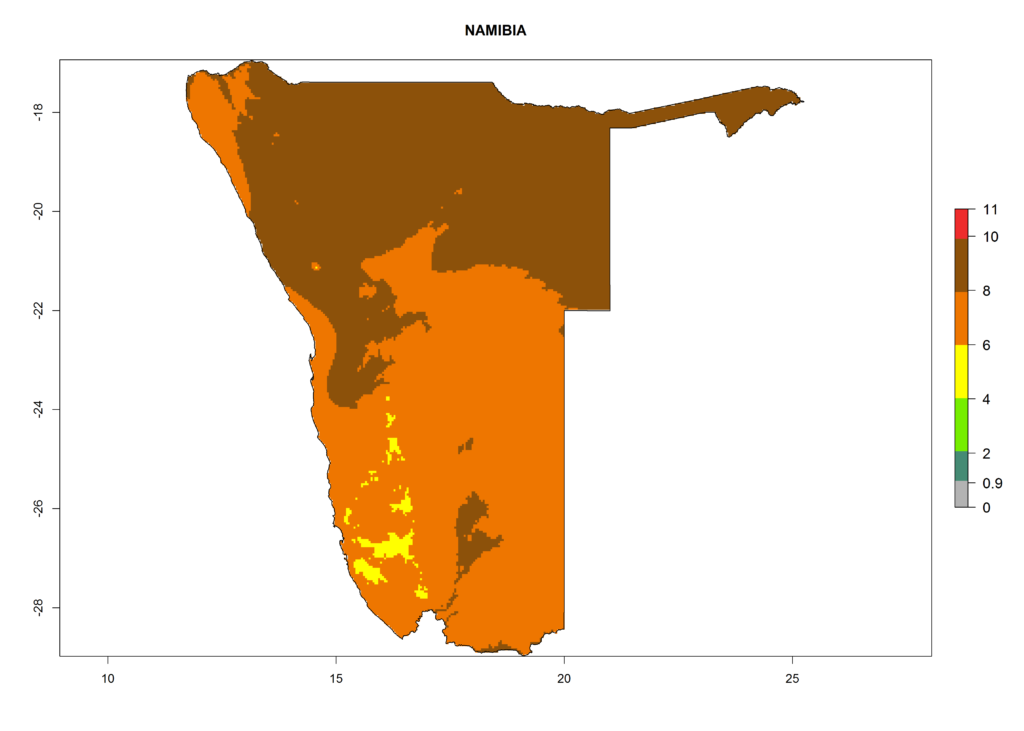
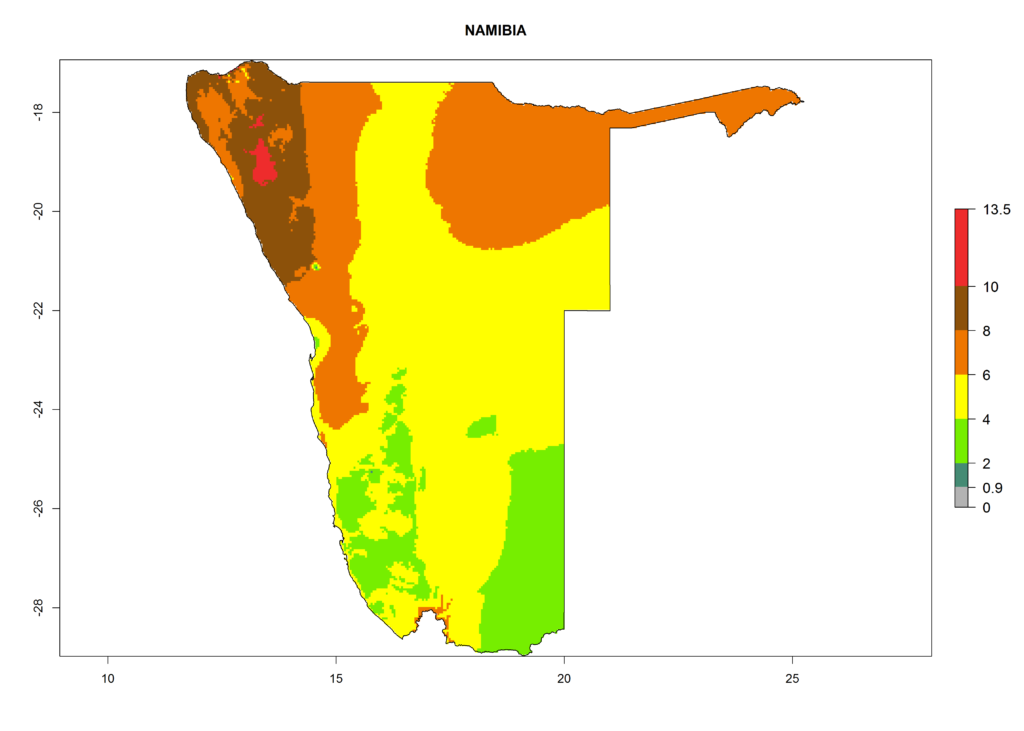
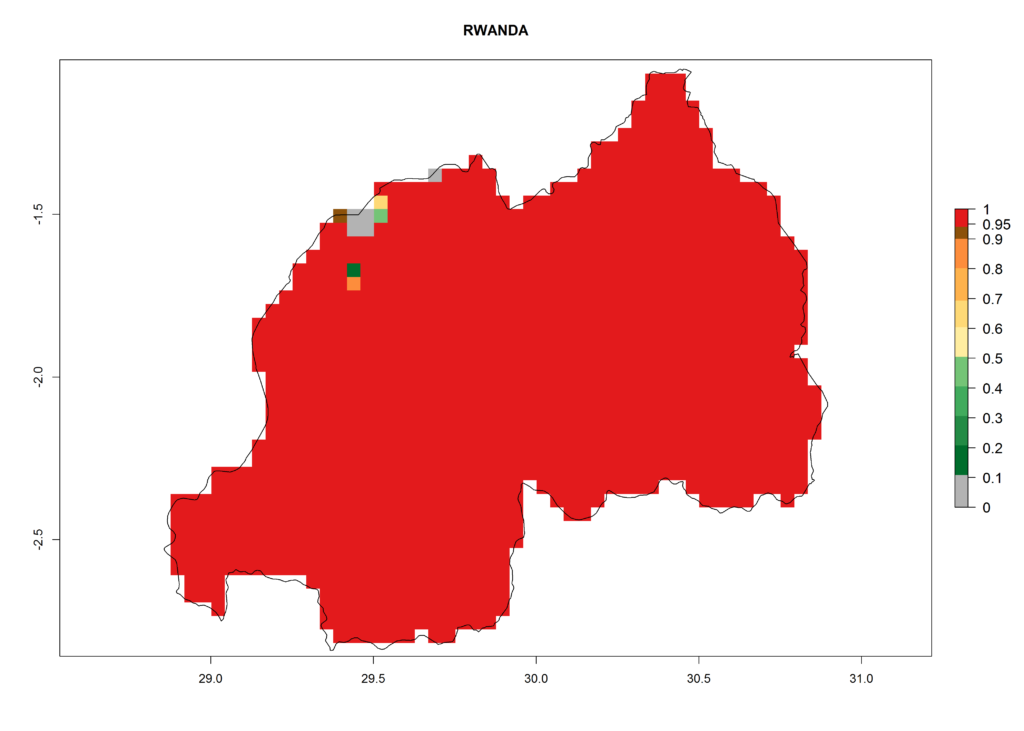
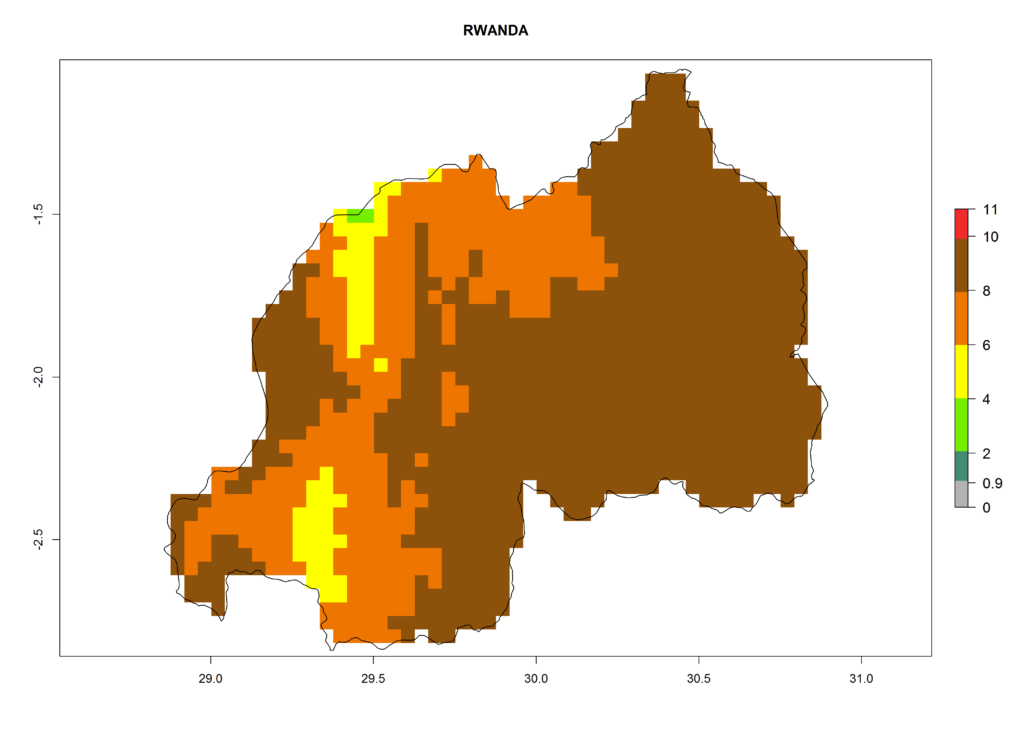
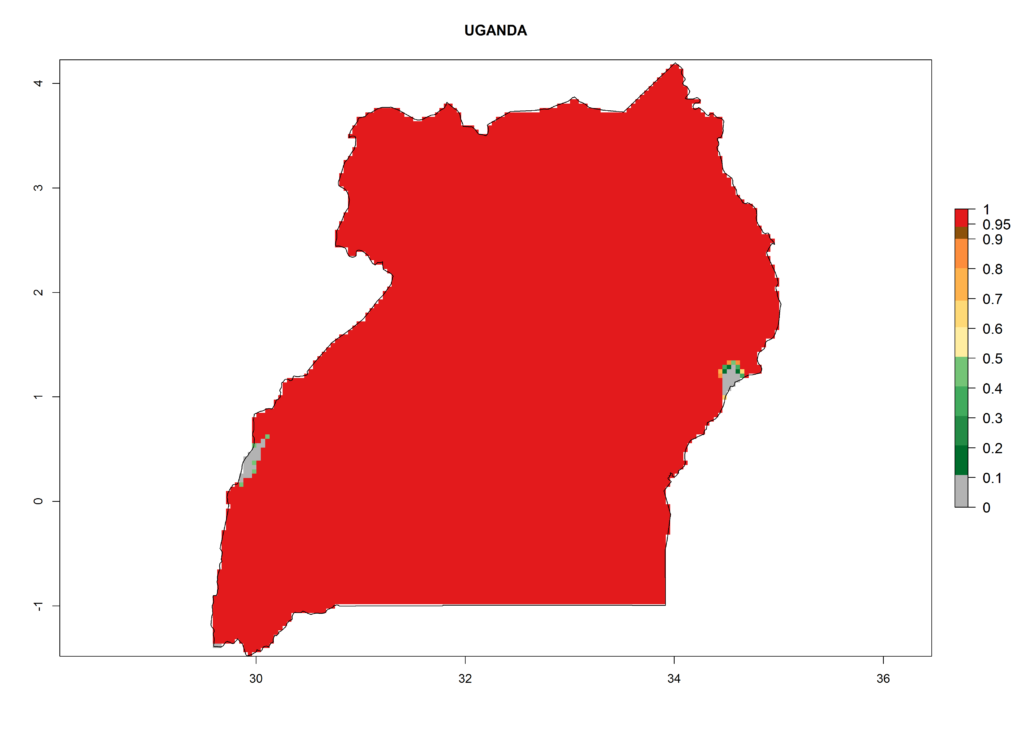
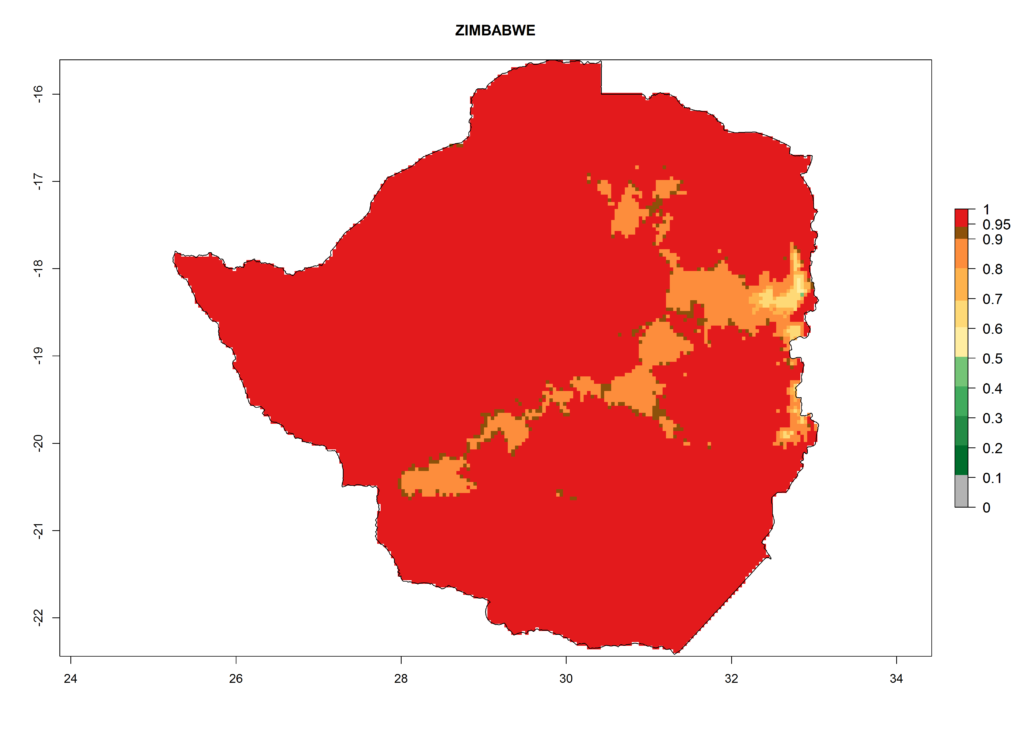
In Africa, T. vaporariorum is already present in Ethiopia, Kenya, Reunion, Zimbabwe, and Morocco, with ERI>0.95, and South Africa, with ERI>0.8 (Fig. 4A). Under the year 2050 temperature scenario, the risk of establishment is predicted to increase in some countries of Southern Africa (Namibia, South Africa, Zimbabwe), so T. vaporariorum will remain a potential risk and threat for food production in countries in Southern and East Africa. These areas are important for the production of food crops such as cassava and tomato, which are affected by viral diseases transmitted by this species, and remain a threat to food production in the countries of eastern South Africa.
Figure 4. Changes in establishment and potential distribution of the greenhouse whitefly, Trialeurodes vaporariorum, in Africa according to model predictions, using the ERI for the years 2000 (A) and 2050 (B), and changes of the ERI between 2000 and 2050 (C). An ERI>0.6 is associated with potential permanent establishment.
Changes in abundance
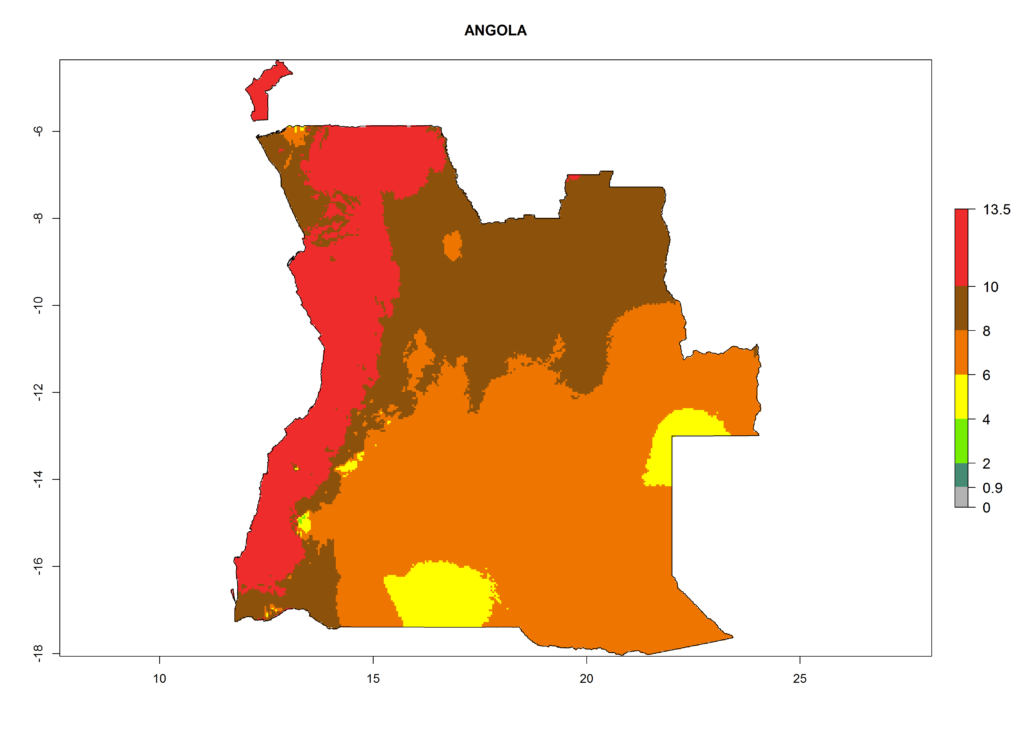
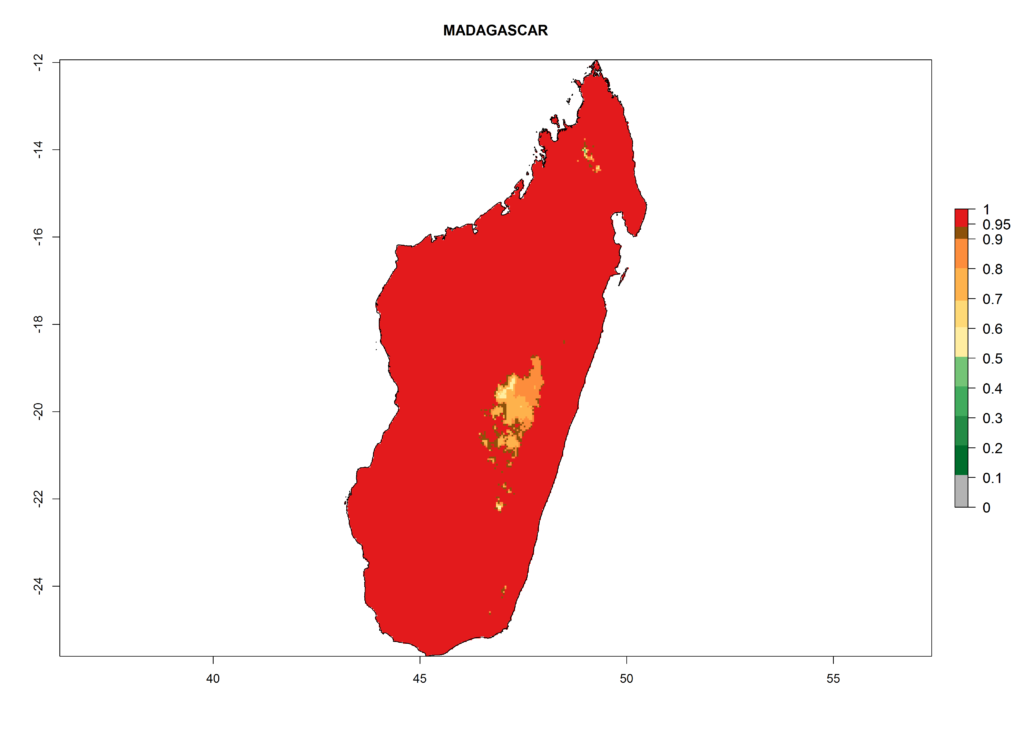
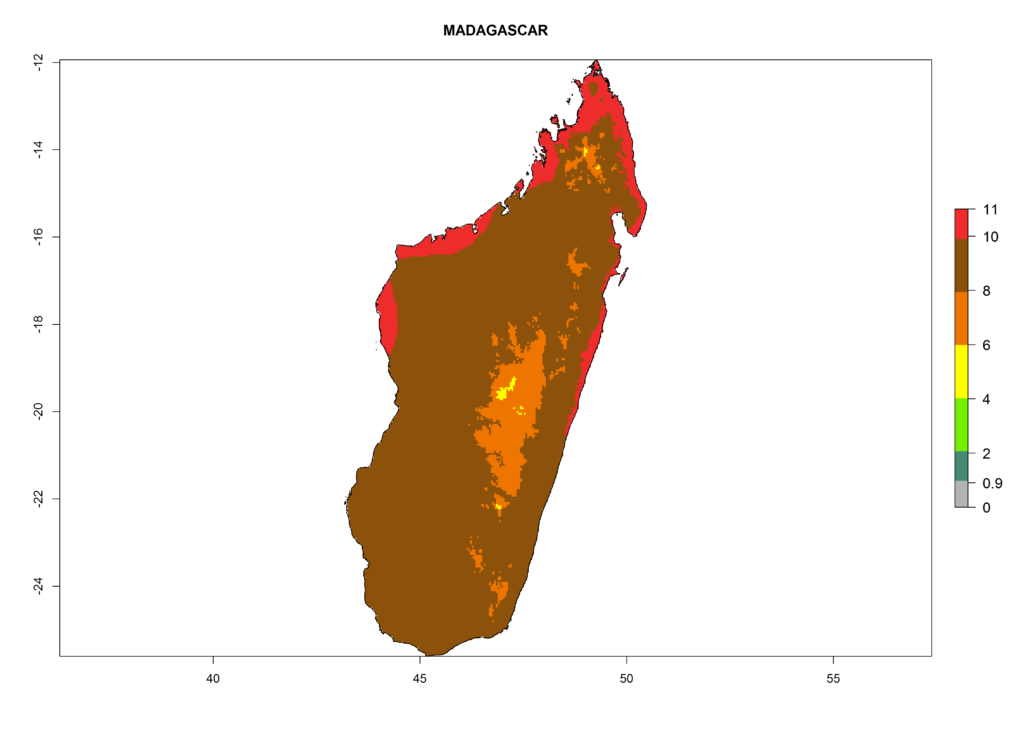
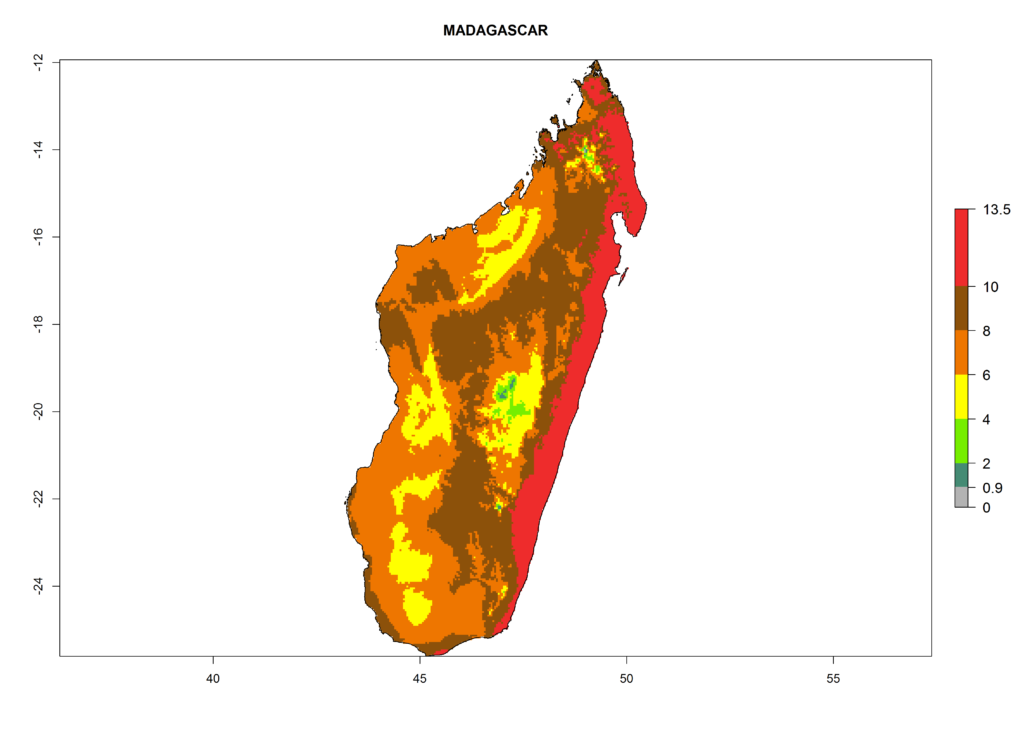
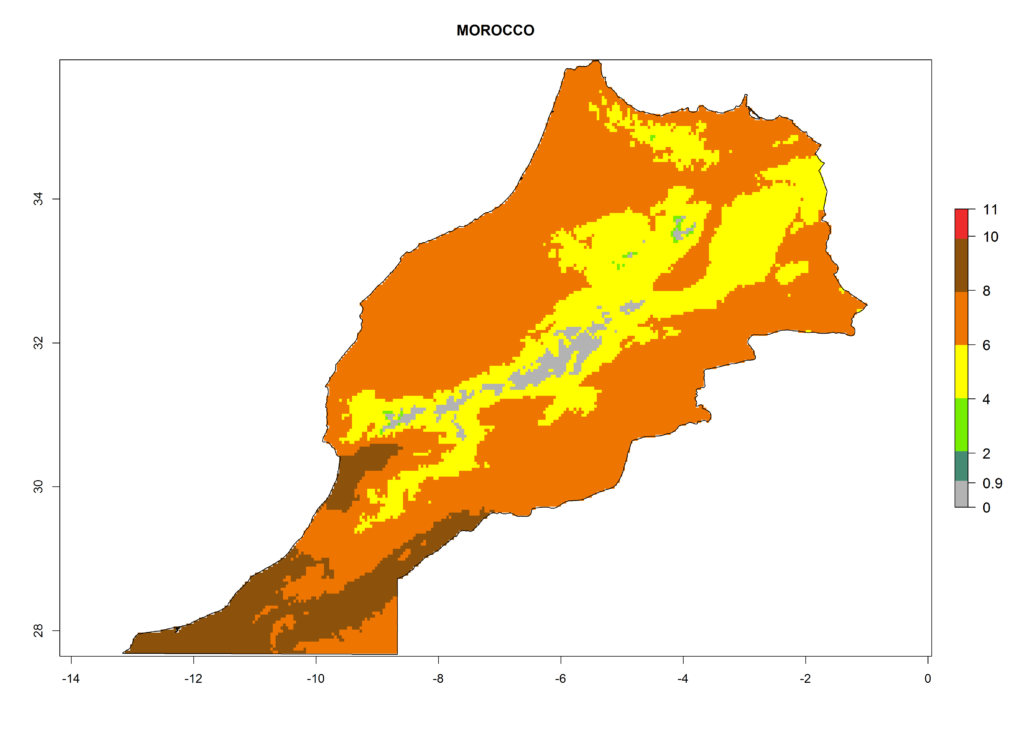
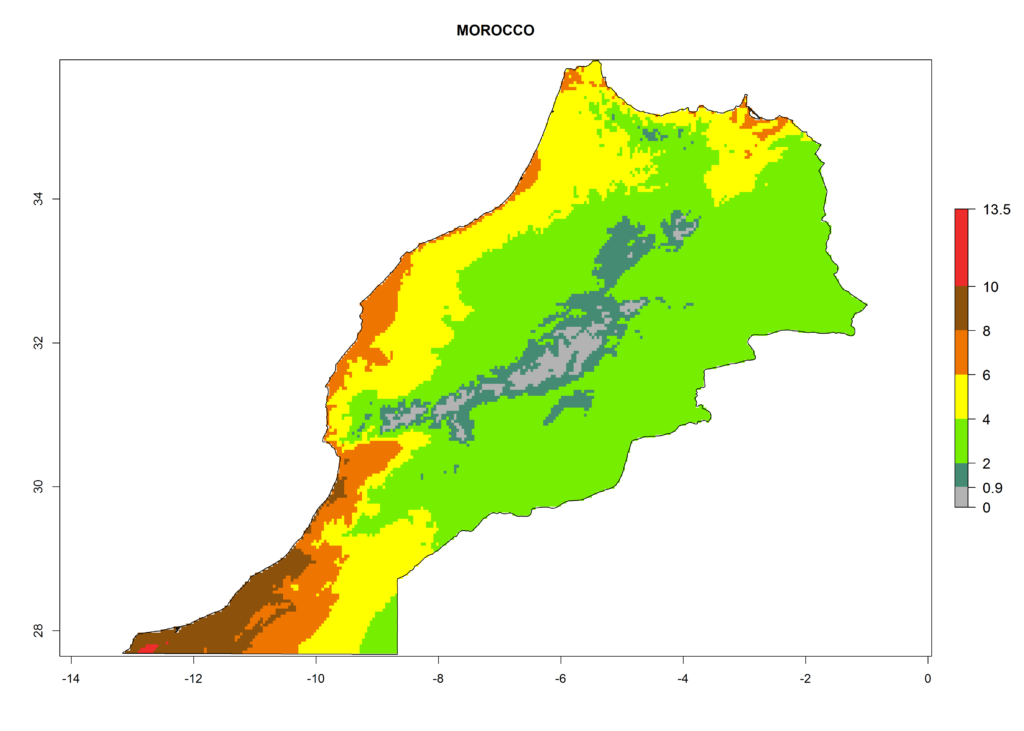
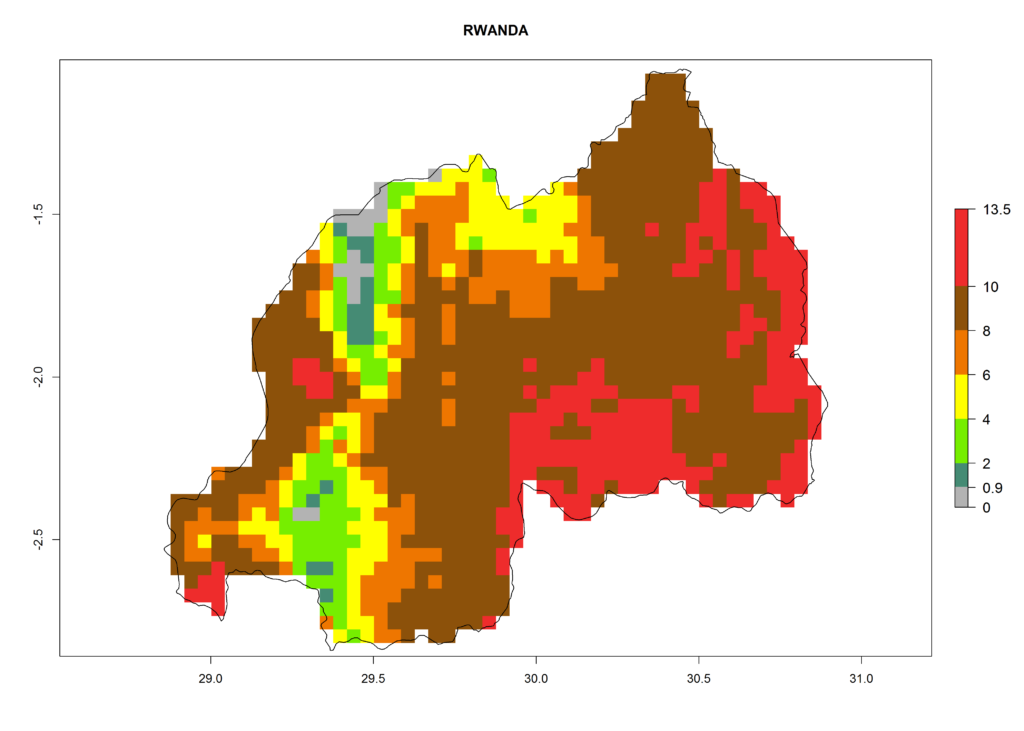
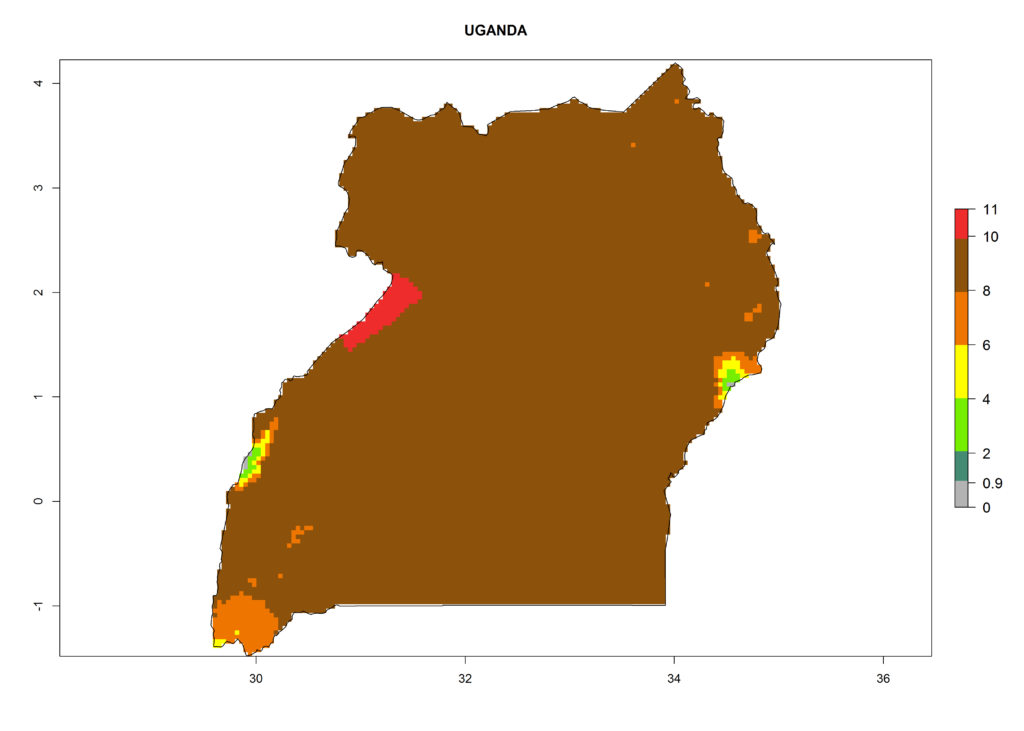
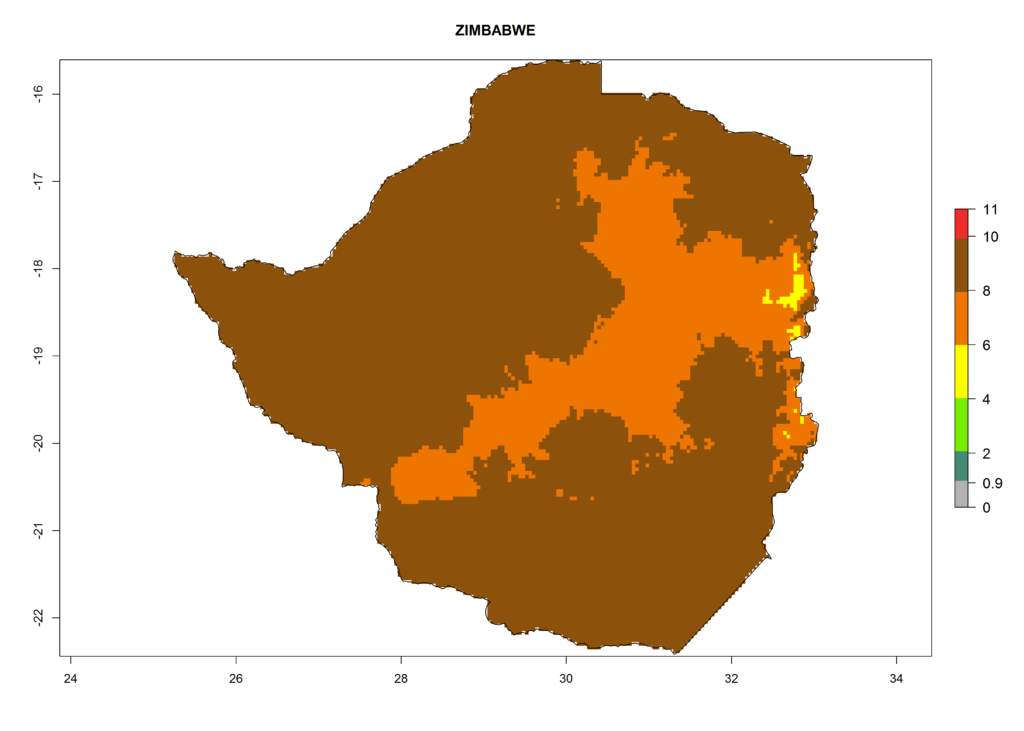
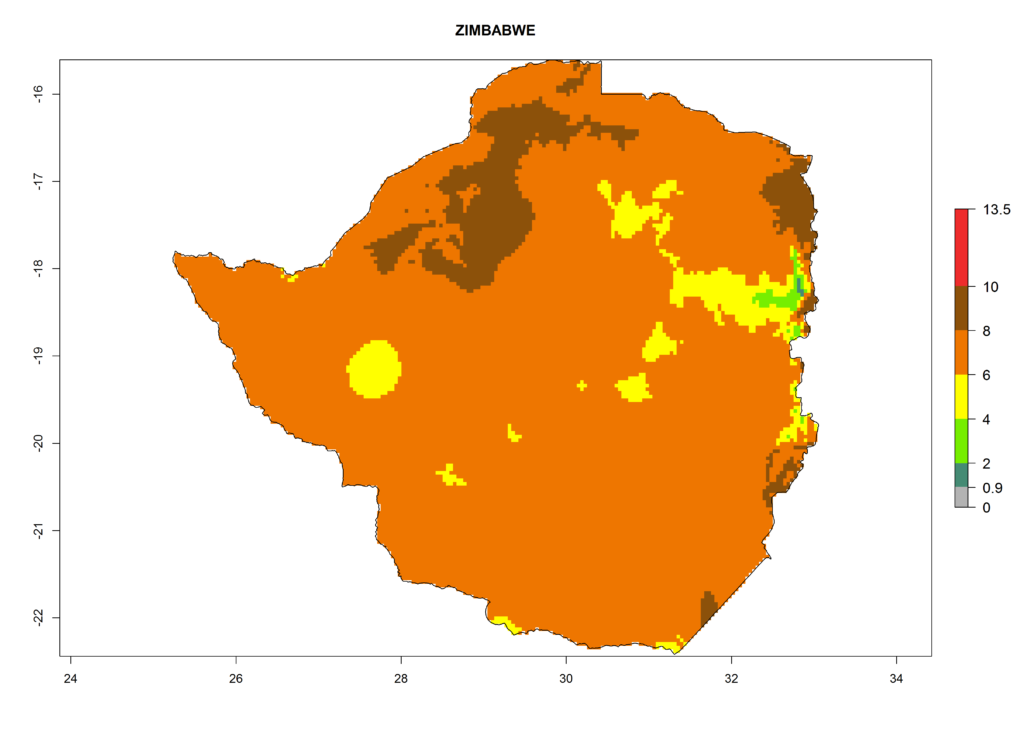
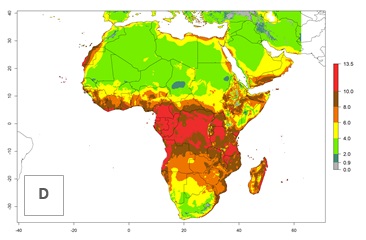
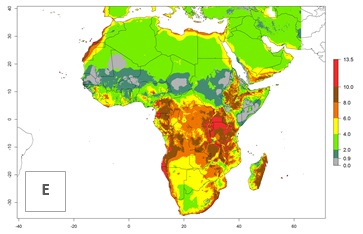
In Africa the highest increase in the number of T. vaporariorum generations (1–2 per year) is predicted for most sub-Saharan countries (Fig. 5C). It is estimated that the change of activity between 2000 and 2050 will be highest in some countries of Southern and East Africa (Fig. 5B, E). An increase in the population growth potential of an AI>1–3 is expected in South Africa, Namibia, Zambia, Angola, Rwanda, Burundi, Uganda, Kenya, Ethiopia, Tanzania, Malawi, and Madagascar (Fig. 5F).
| GI | AI | |
| 2000 | 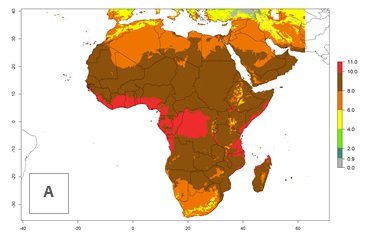 |
 |
| 2050 | 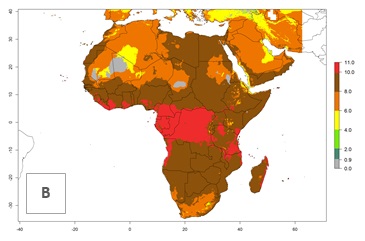 |
 |
| Index change (2000 – 2050) | 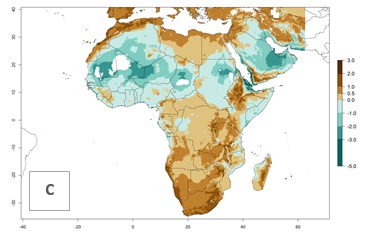 |
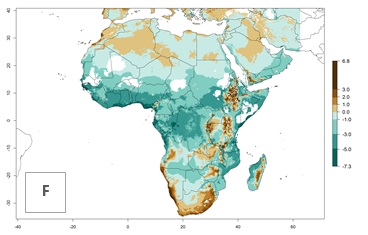 |
Figure 5. Changes in abundance (GI, damage potential) and activity (AI, potential population growth) of the whitefly, Trialeurodes vaporariorum, in Africa according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
Country Risk Maps
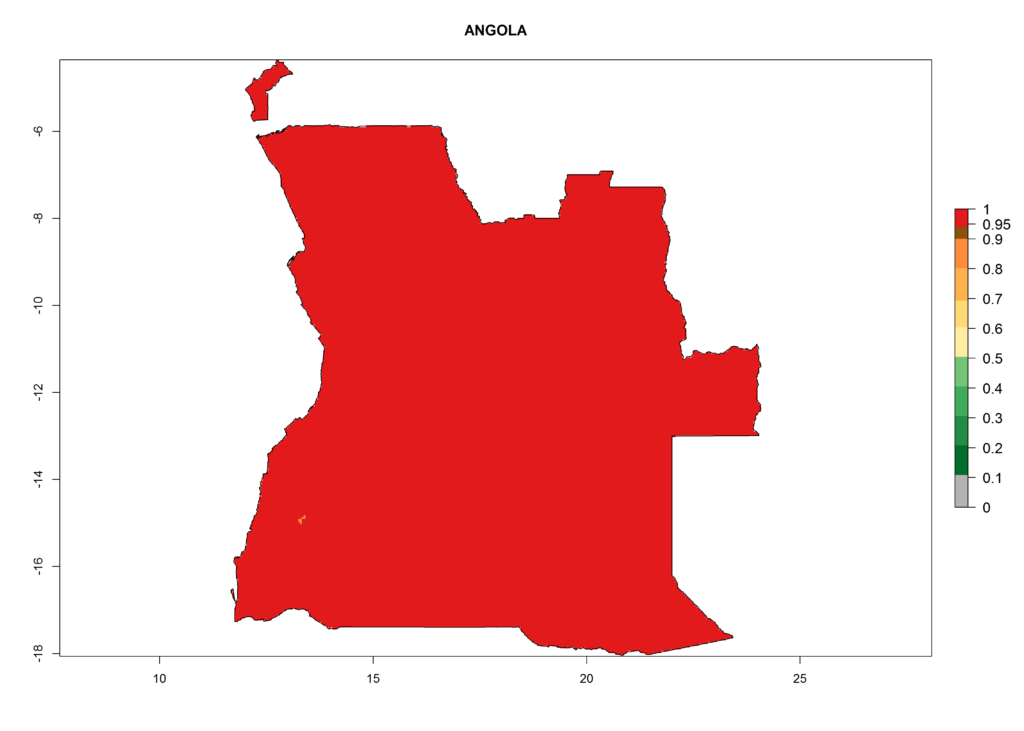
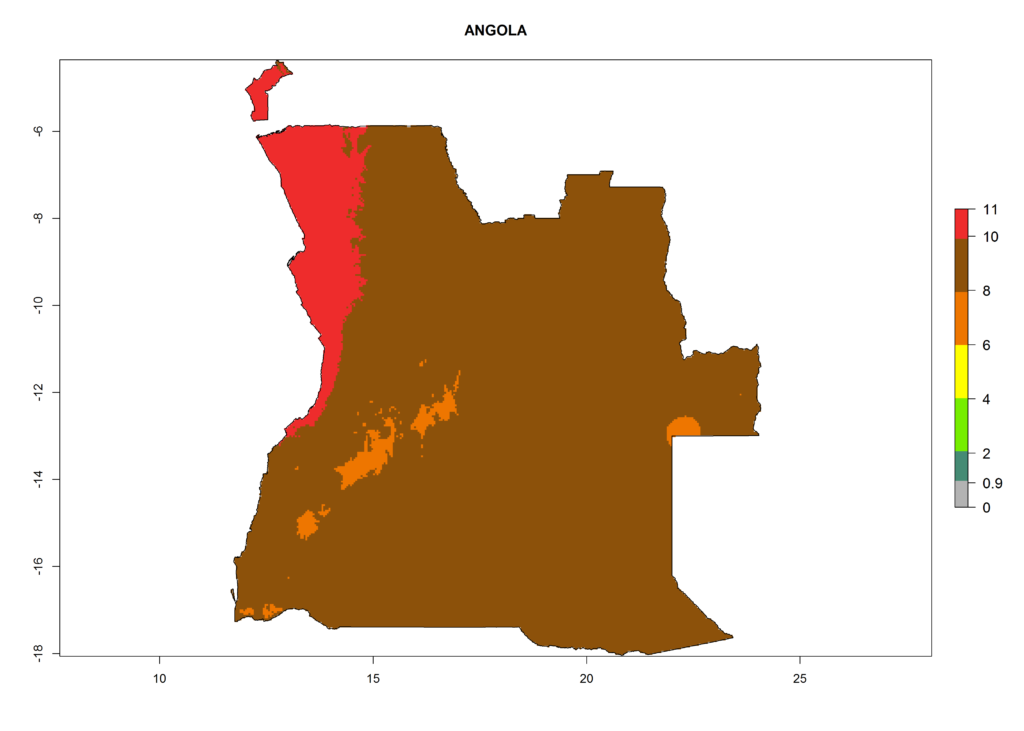
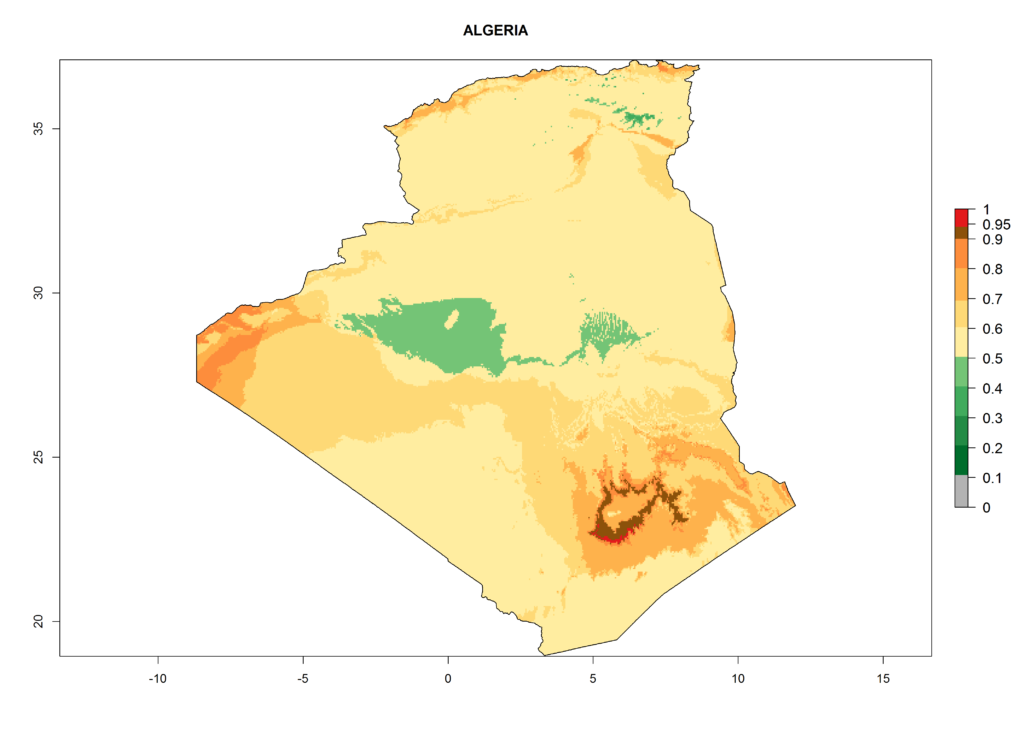
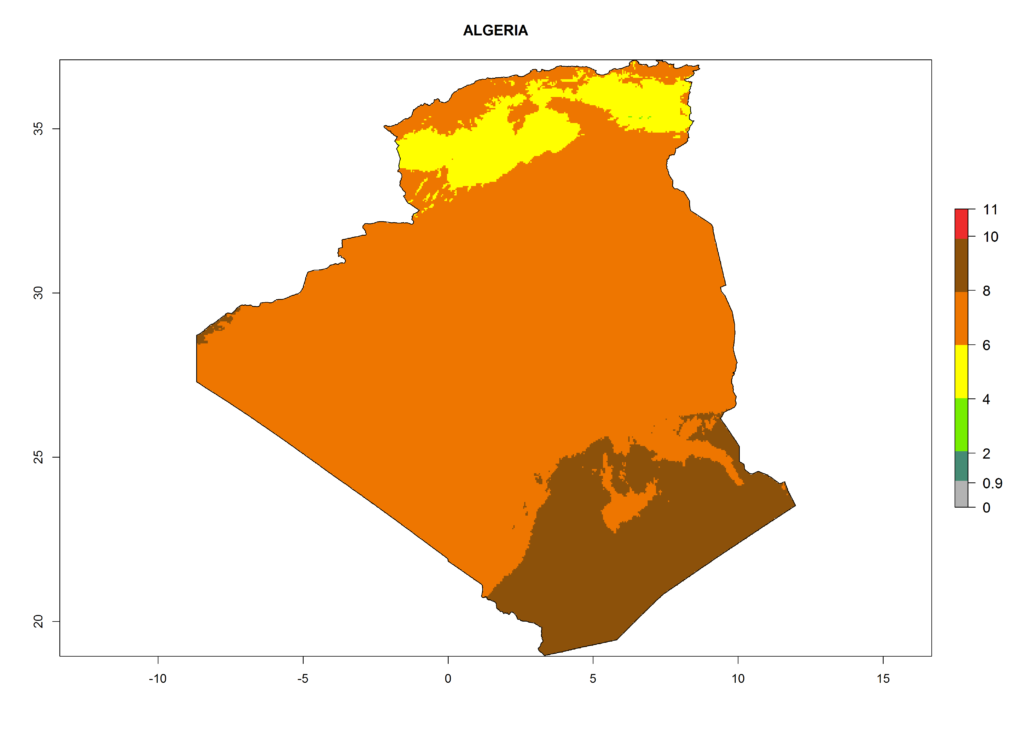
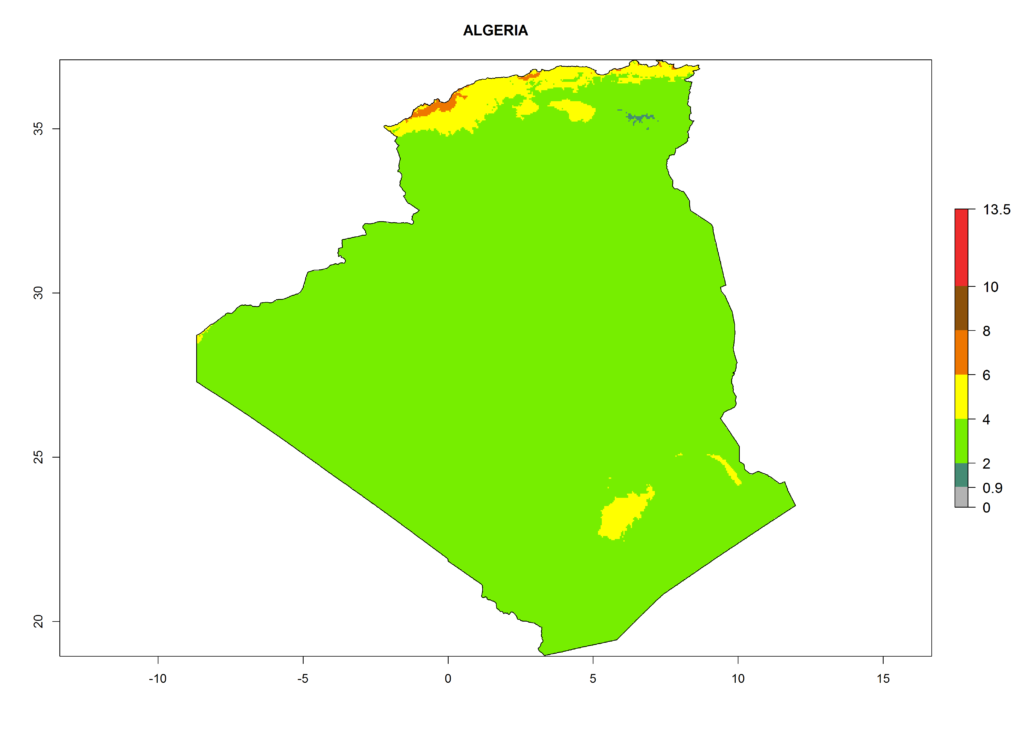
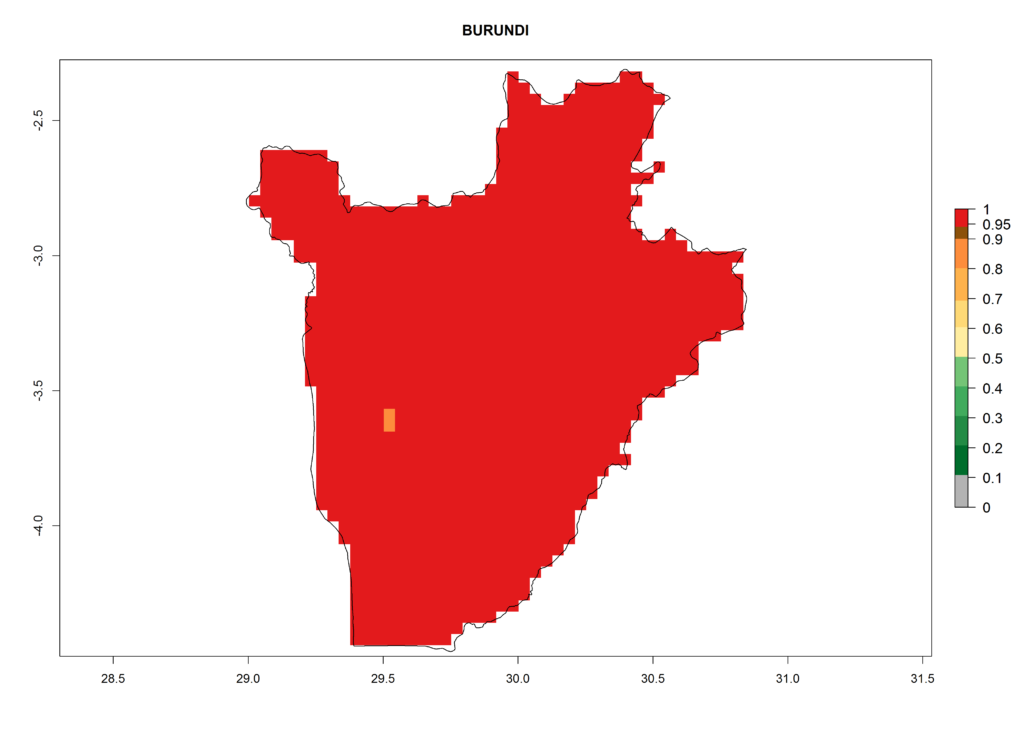
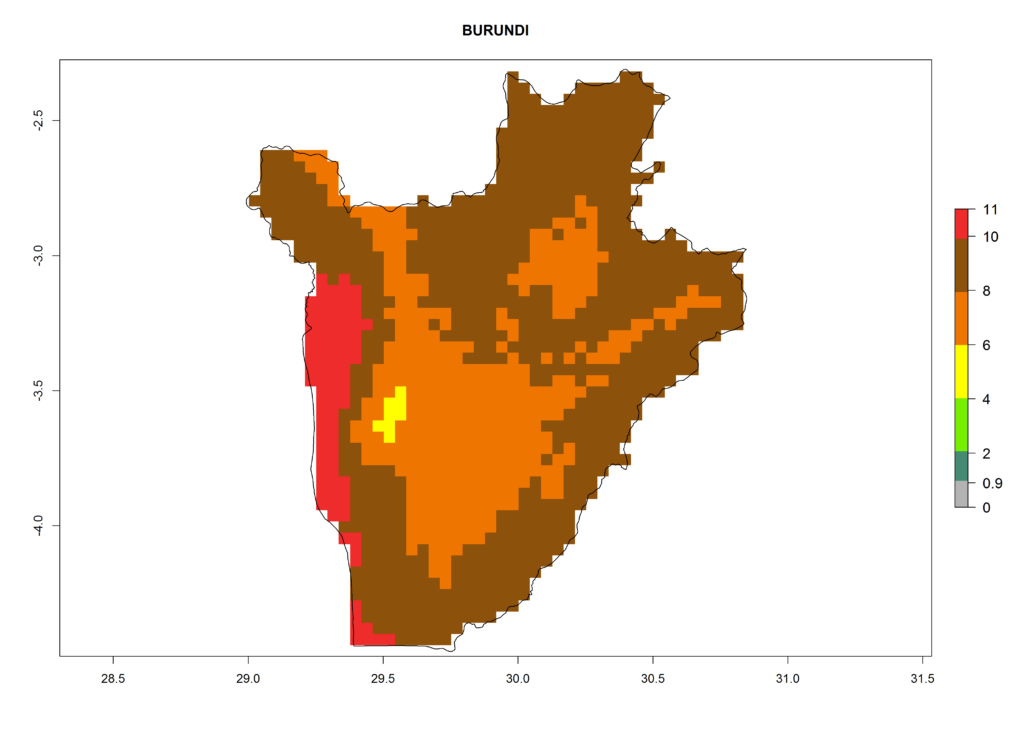
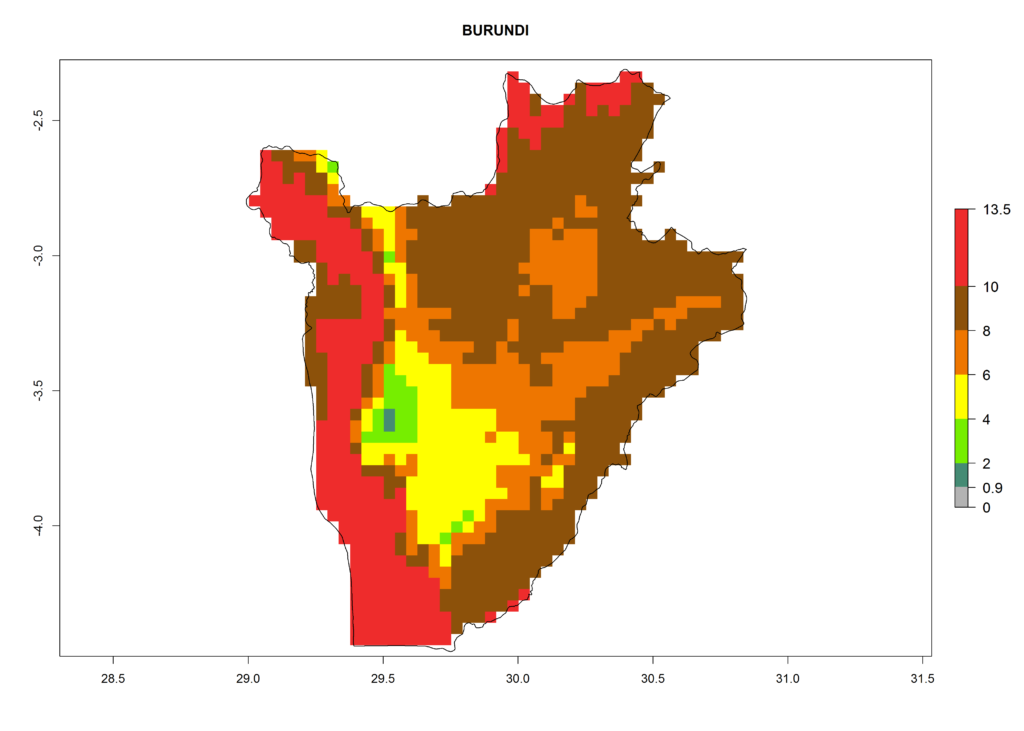
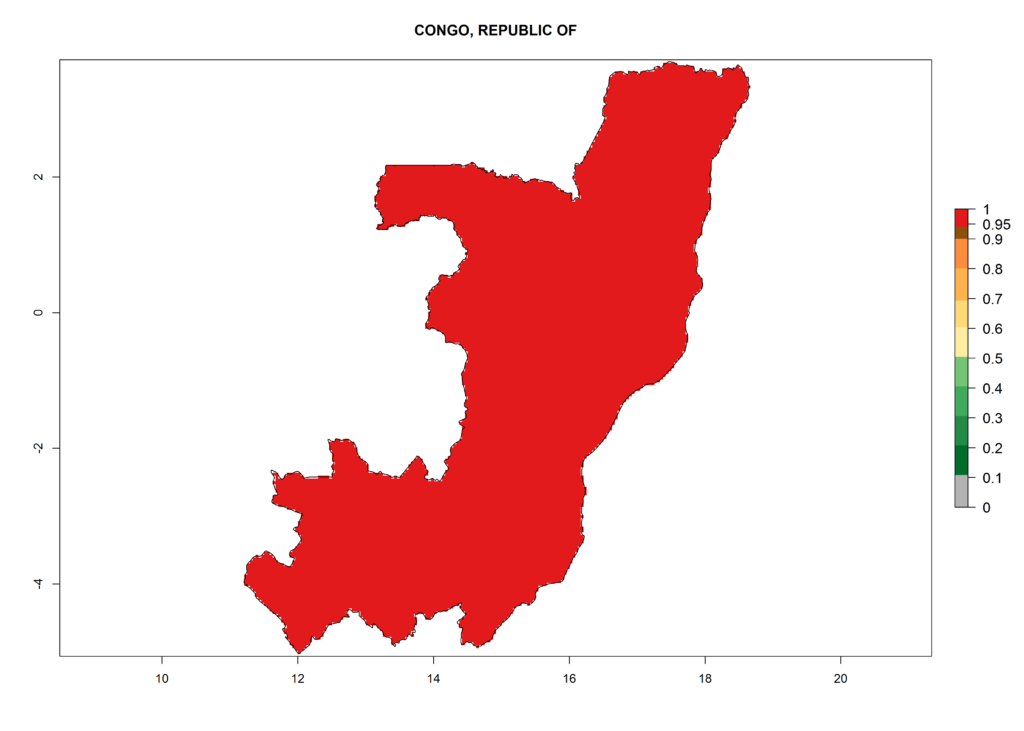
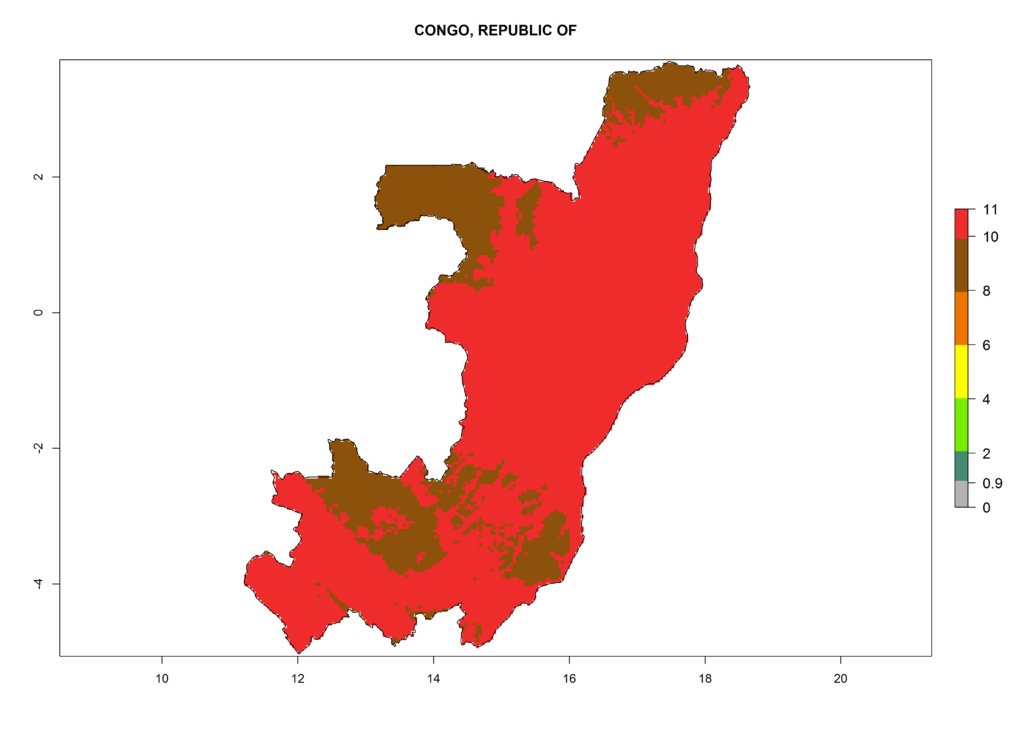
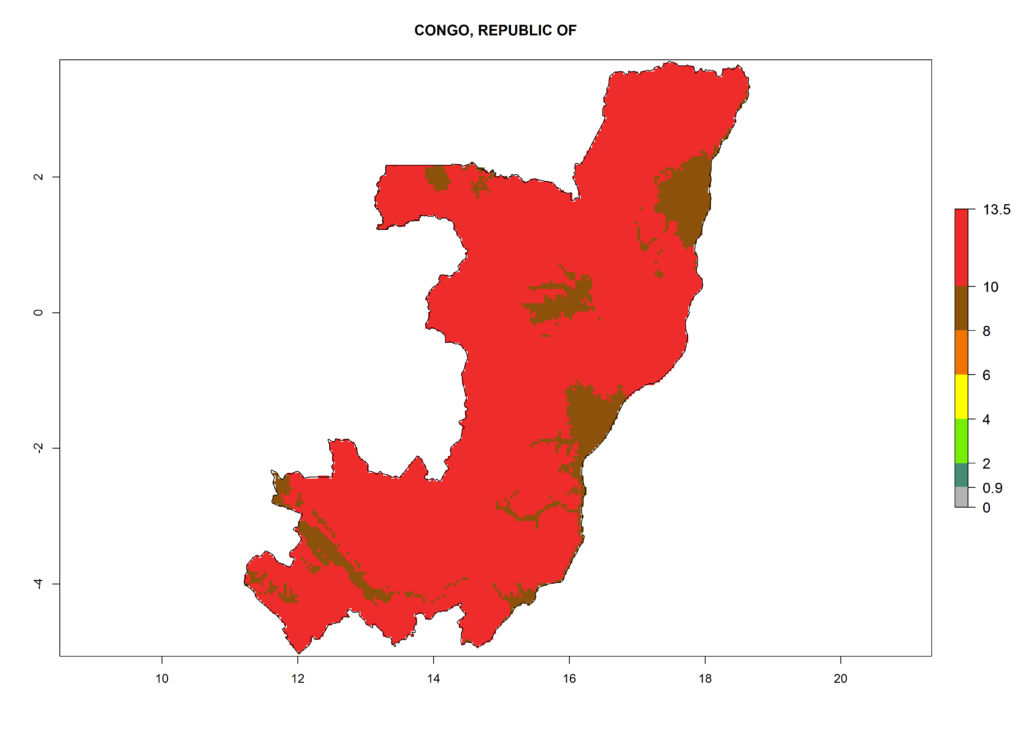
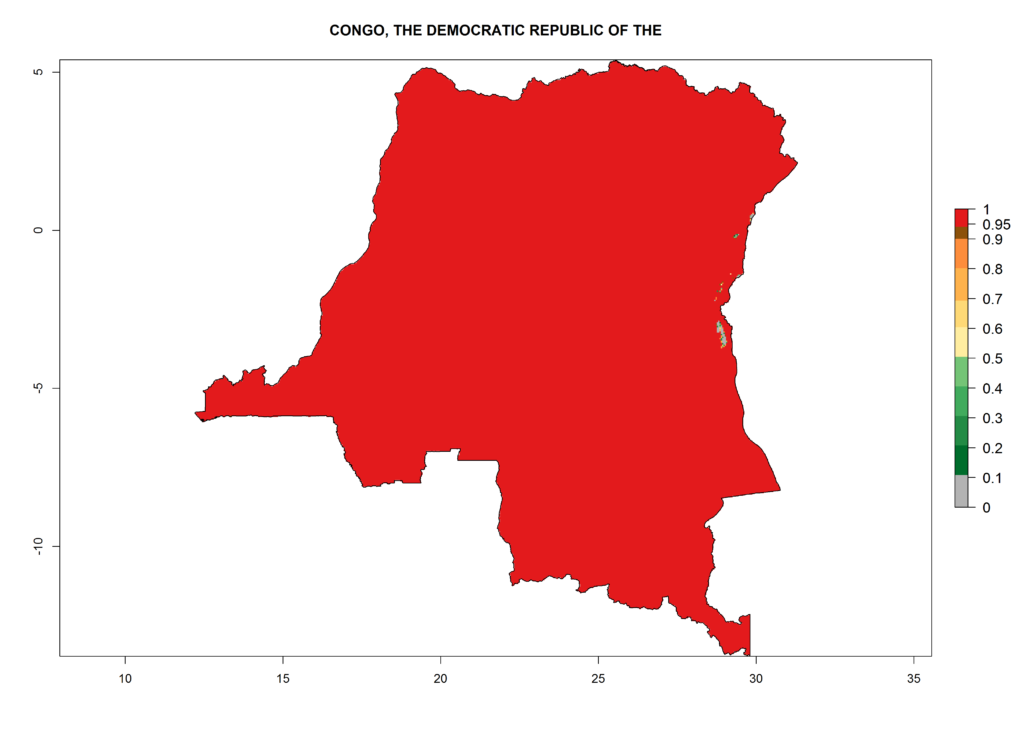
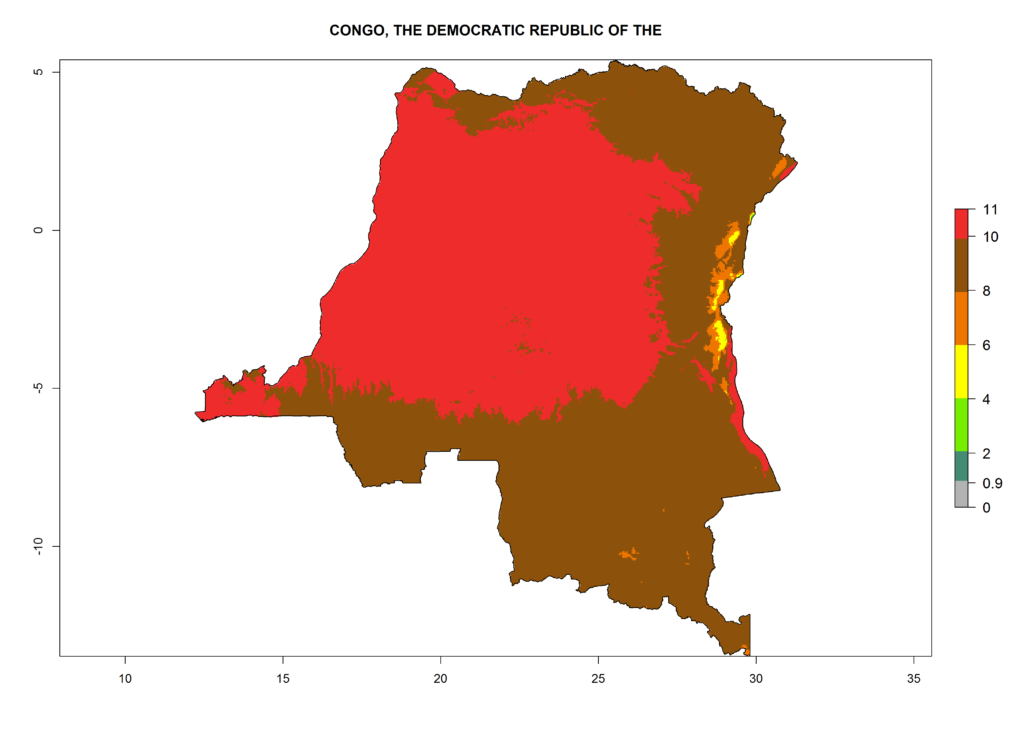
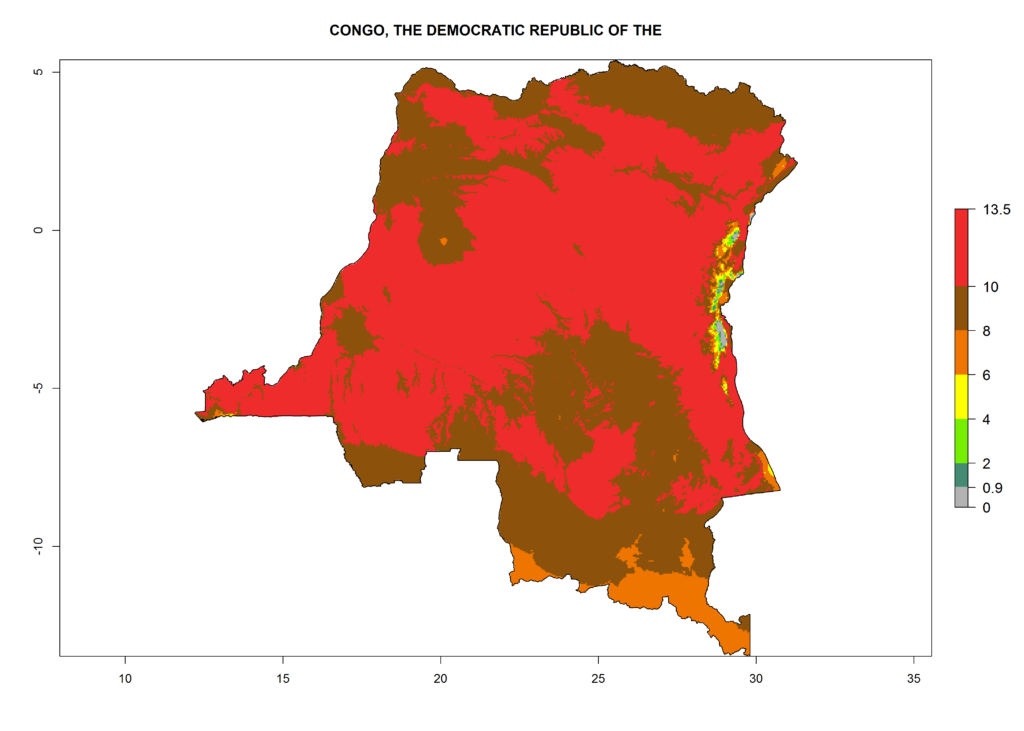
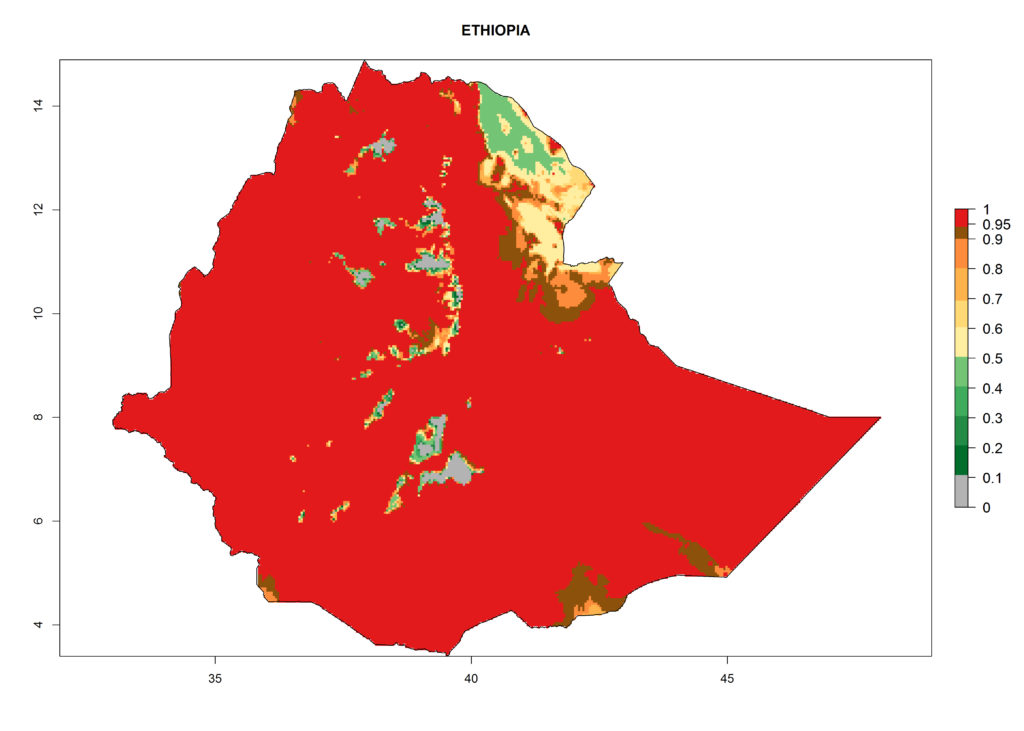
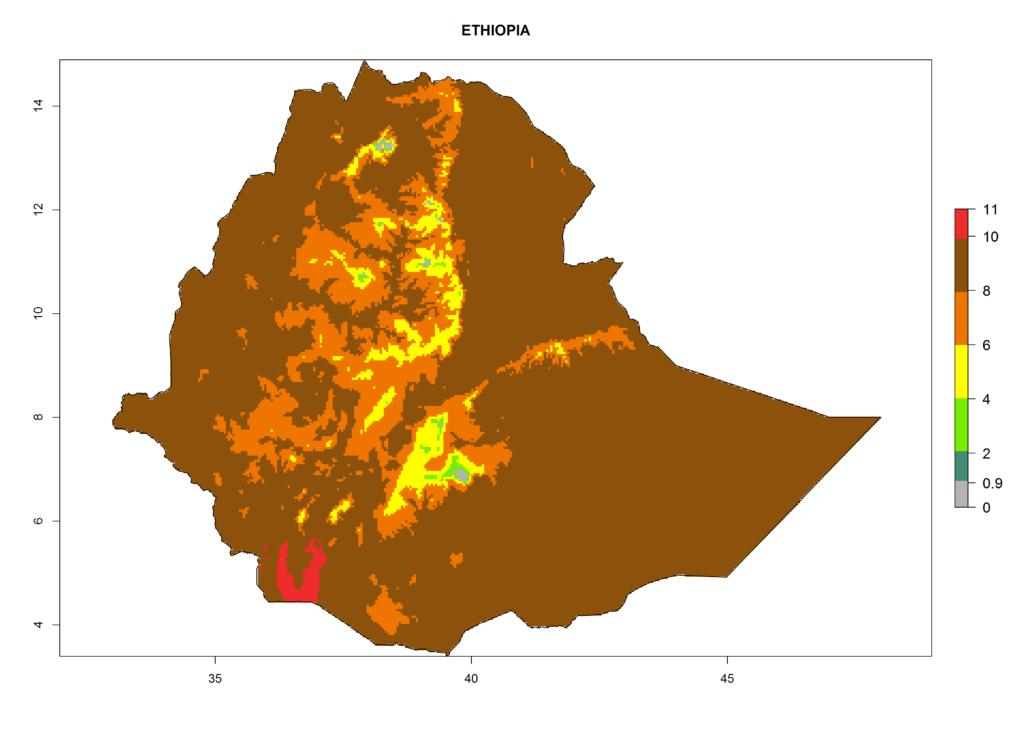
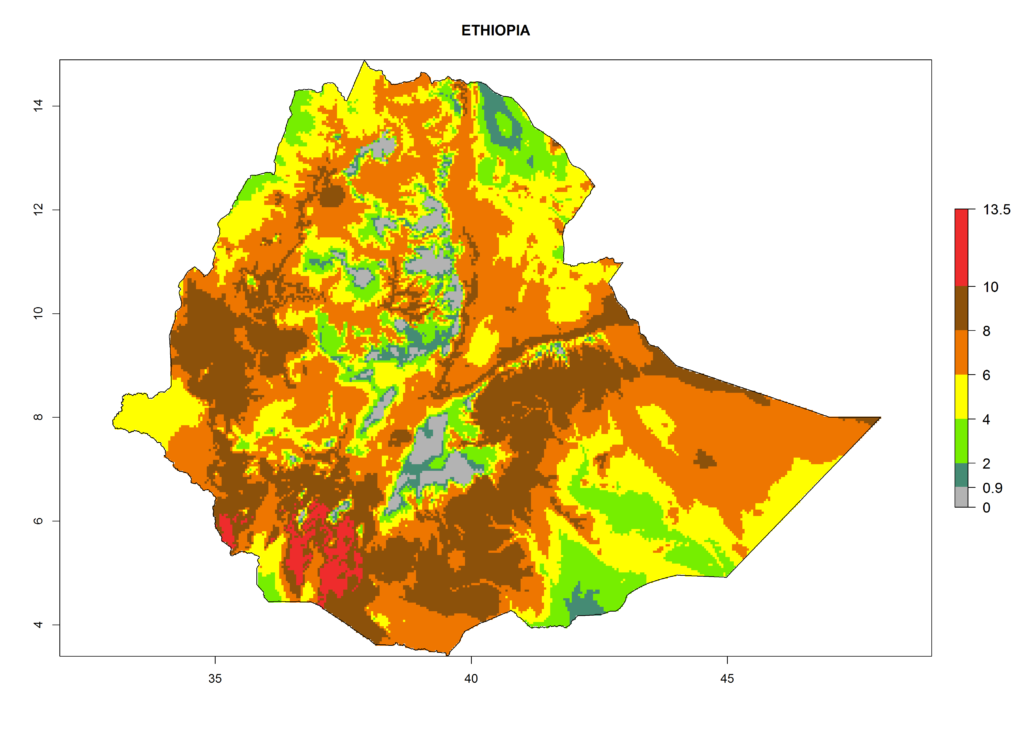
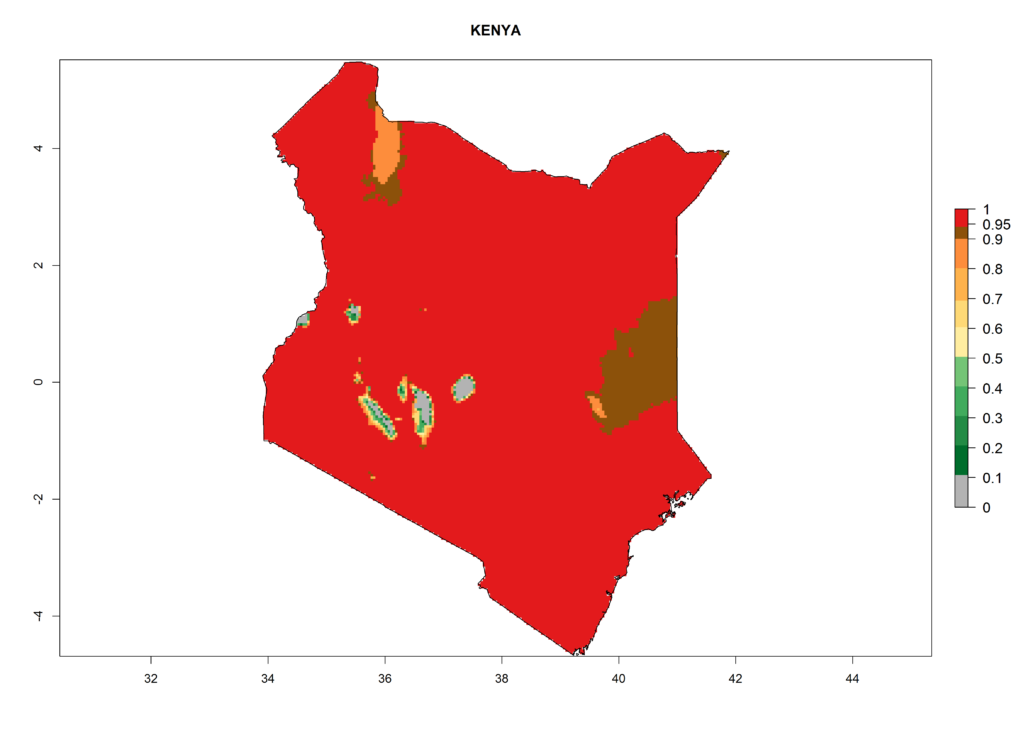
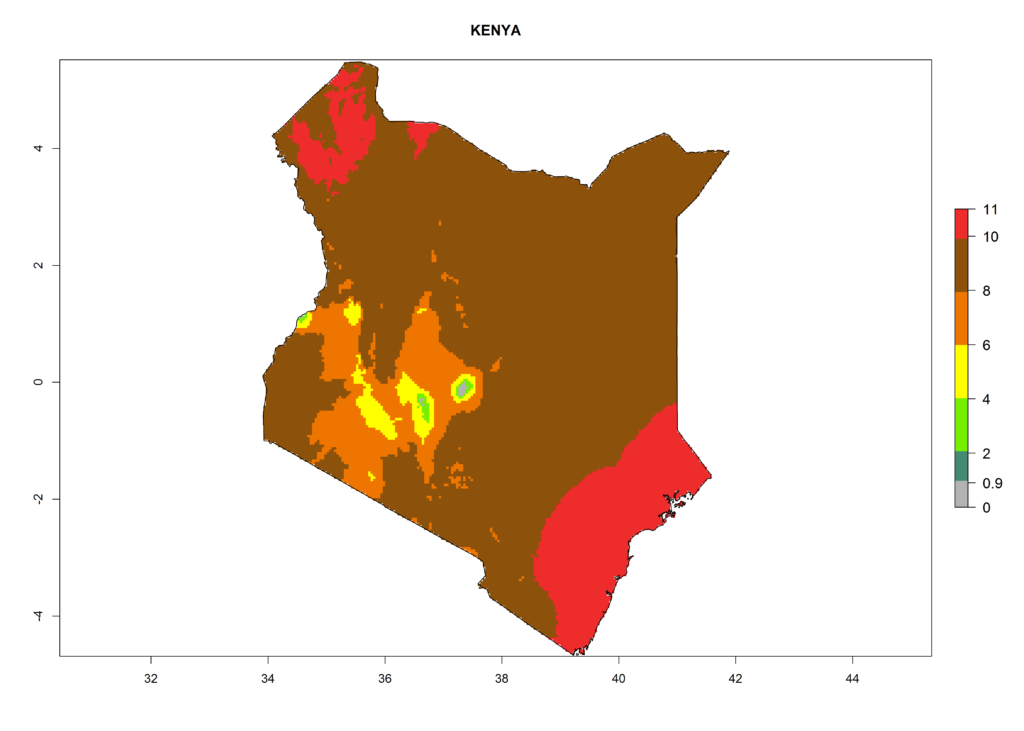
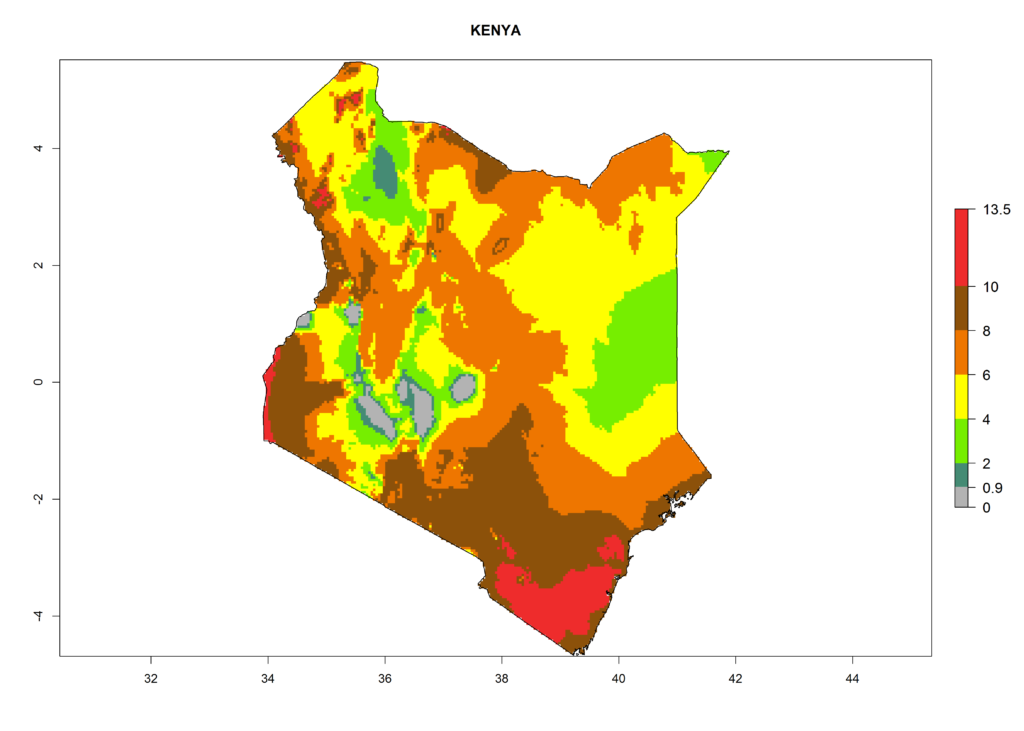
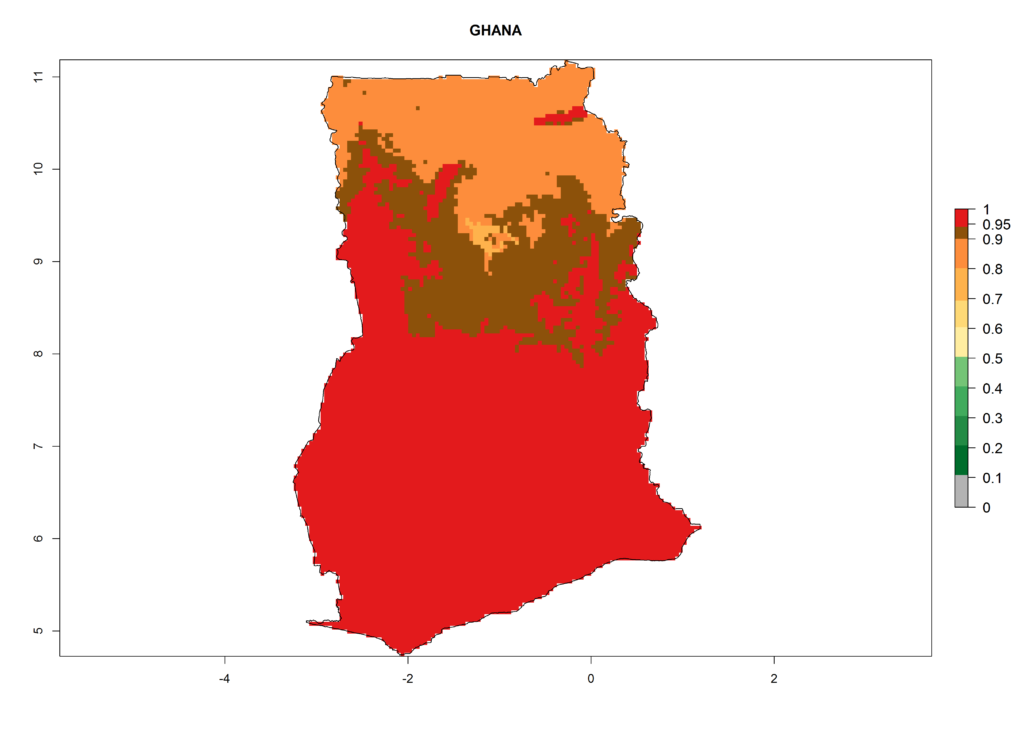
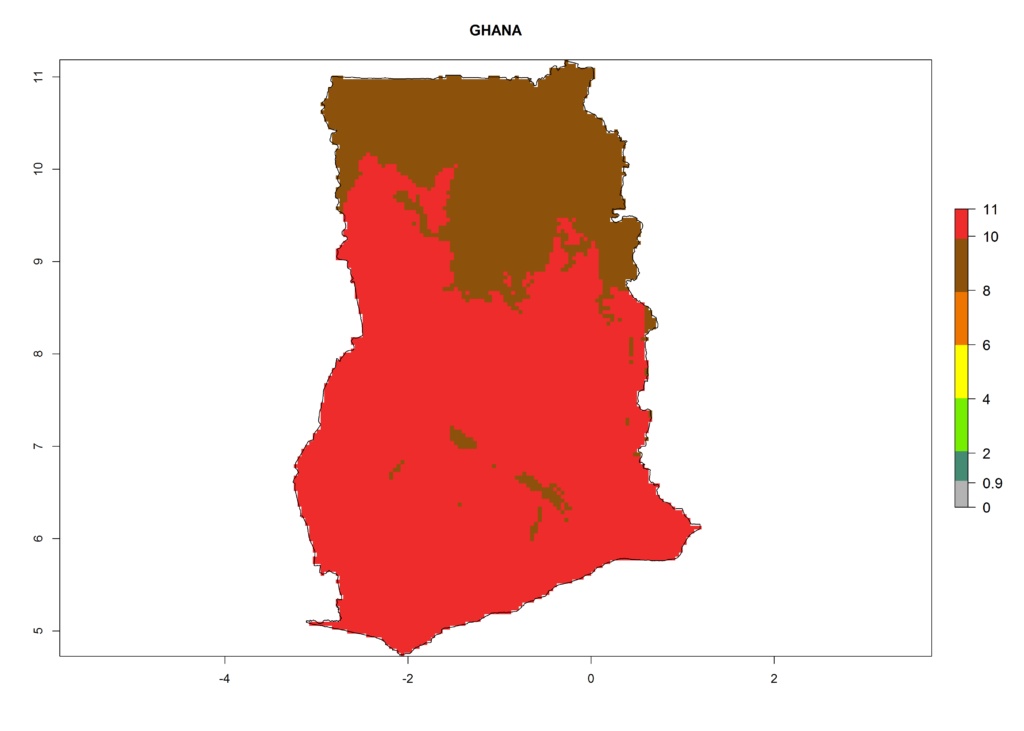
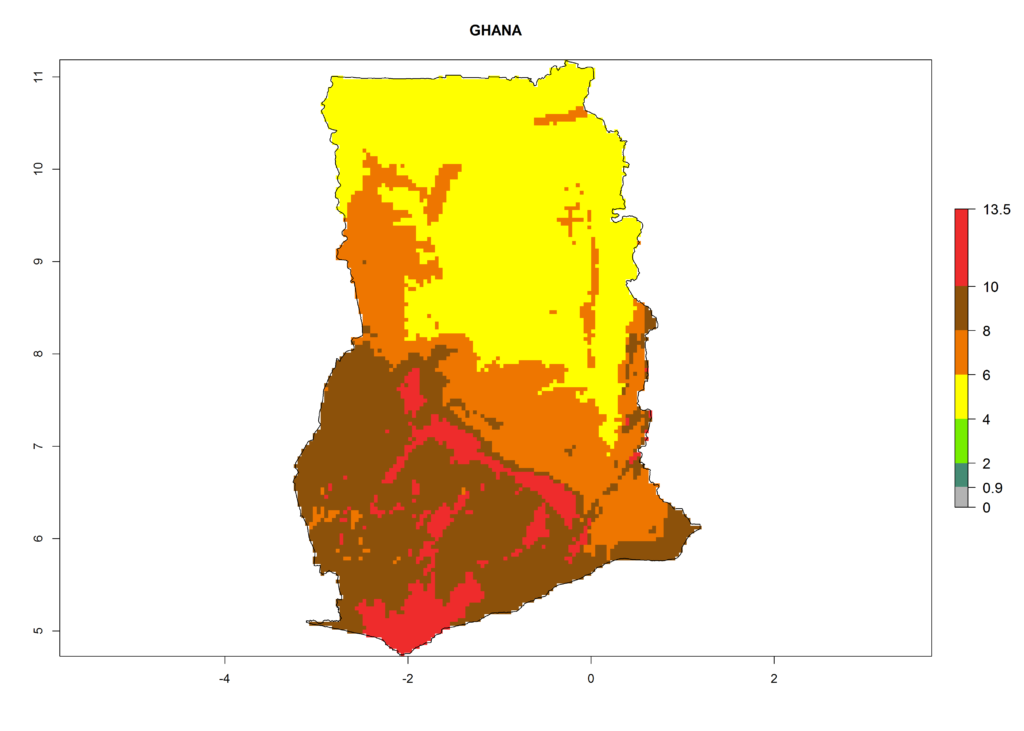
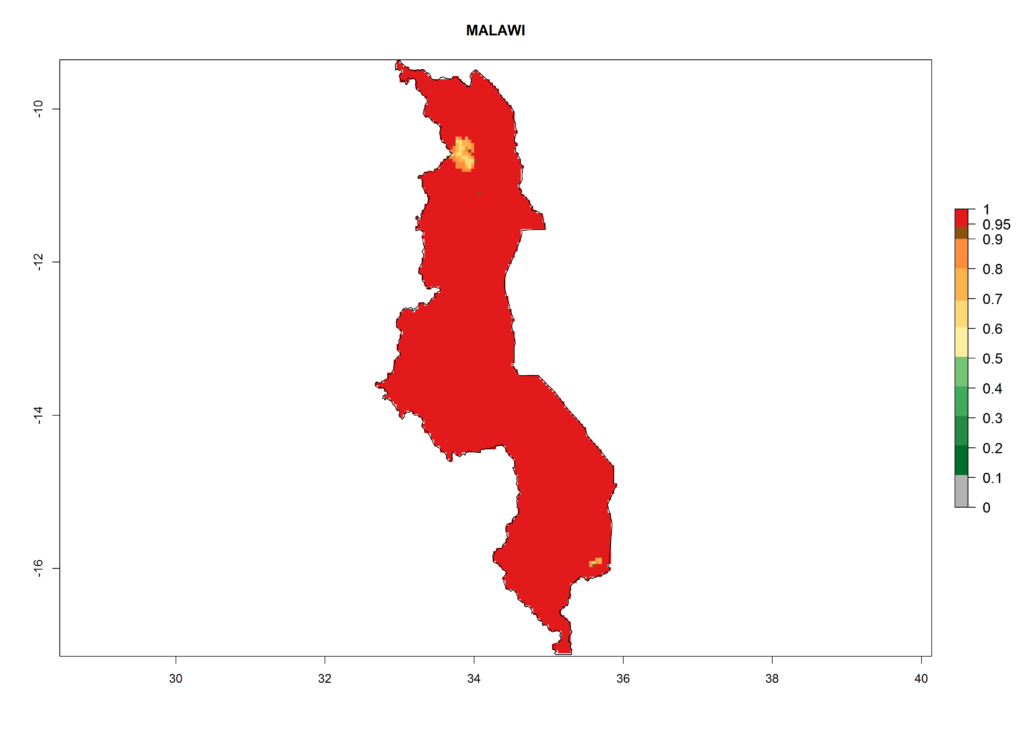
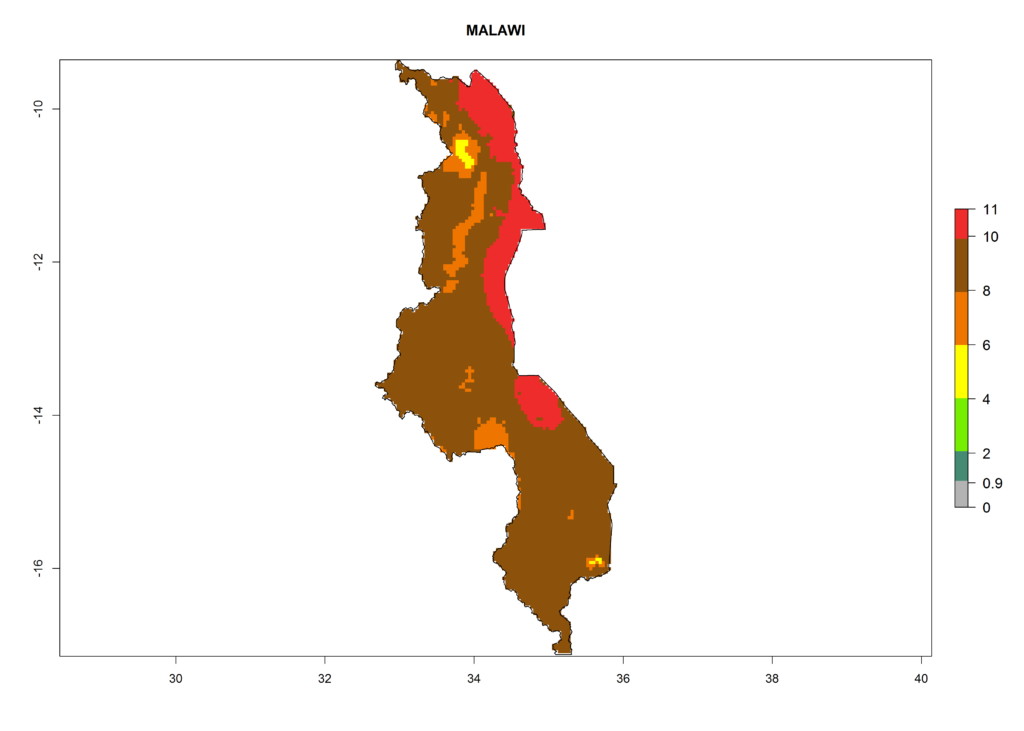
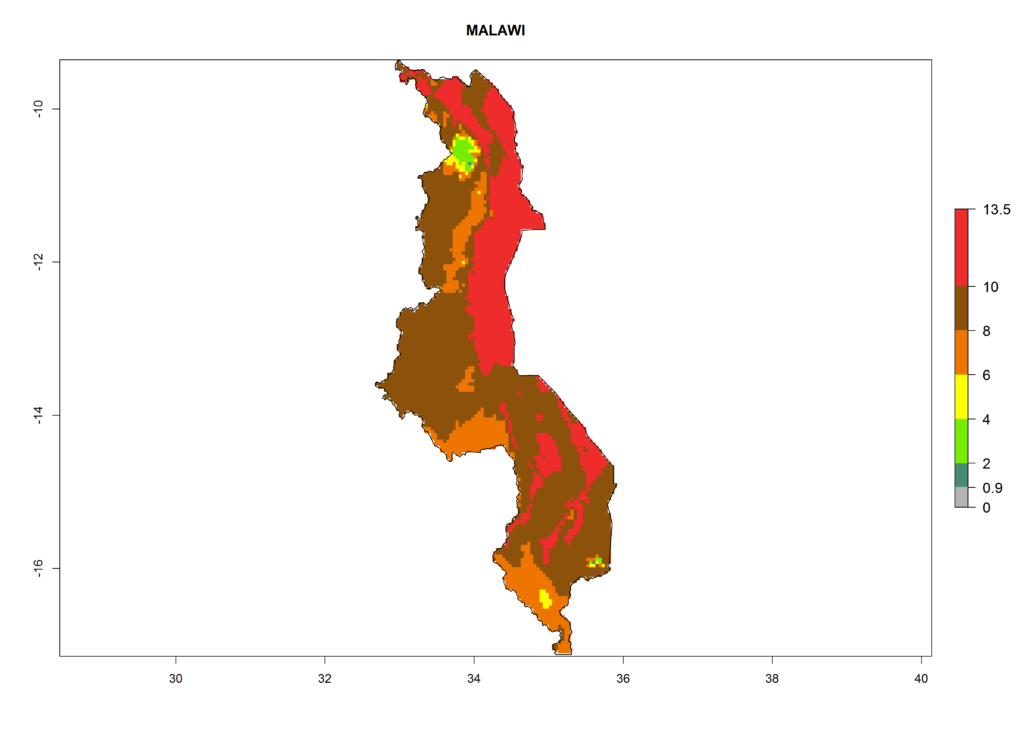
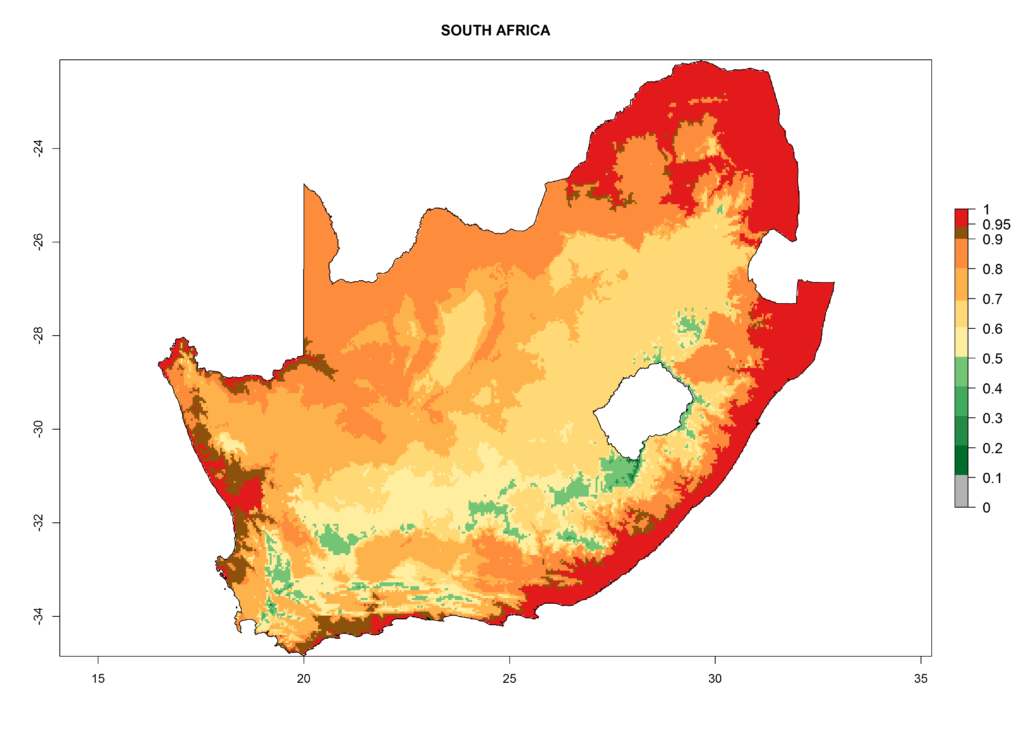
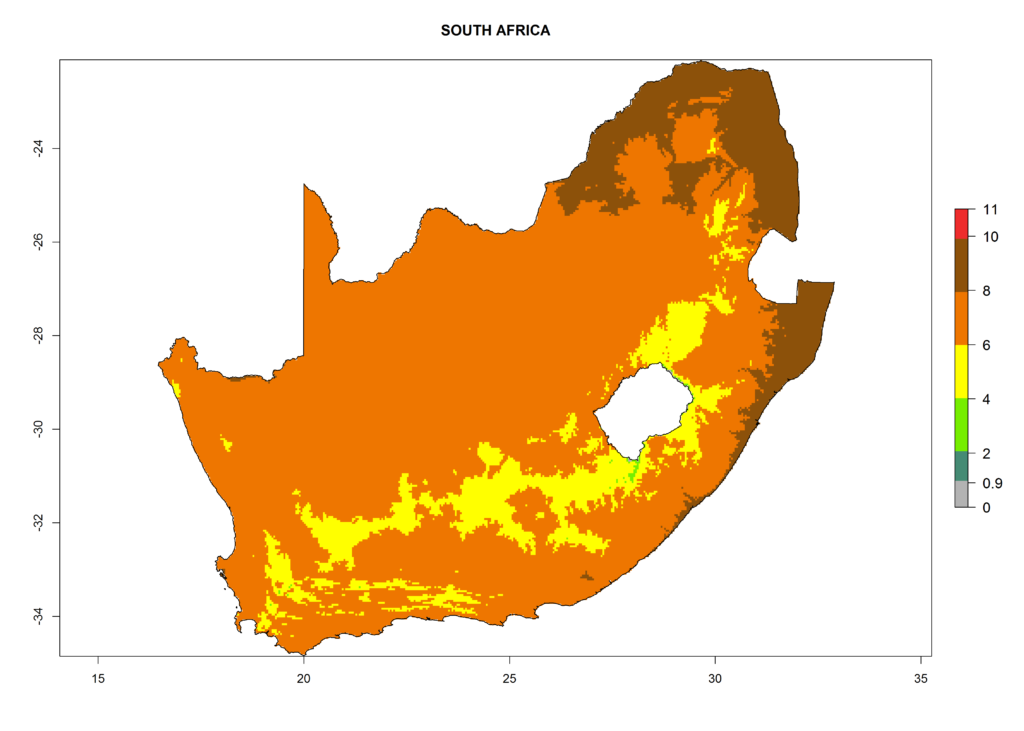
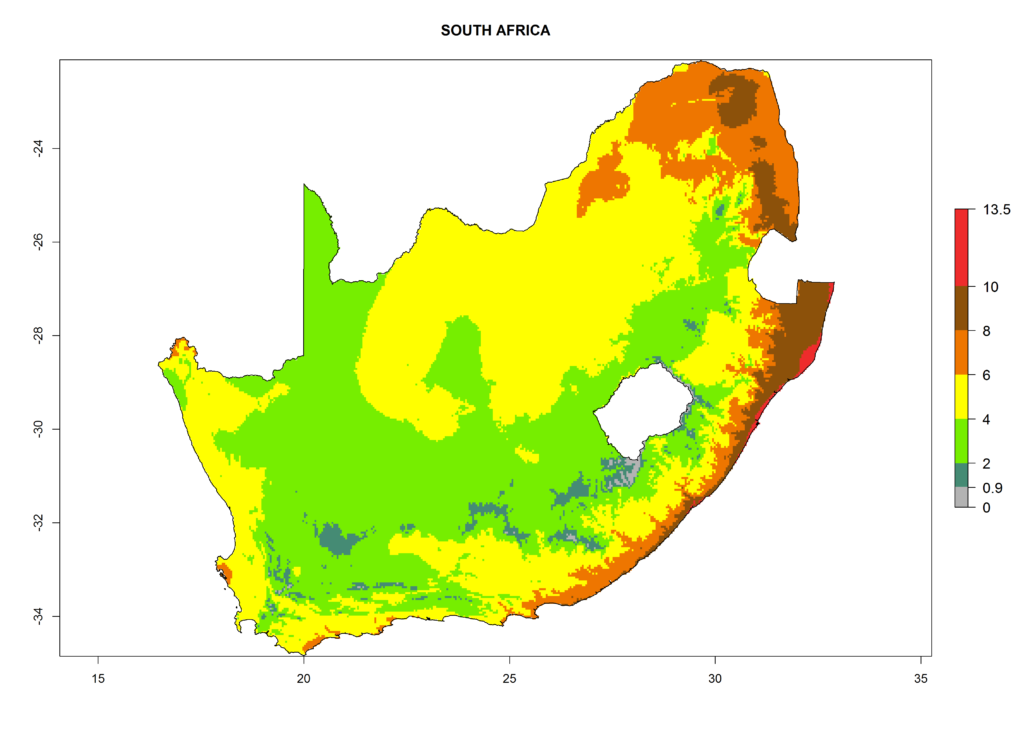
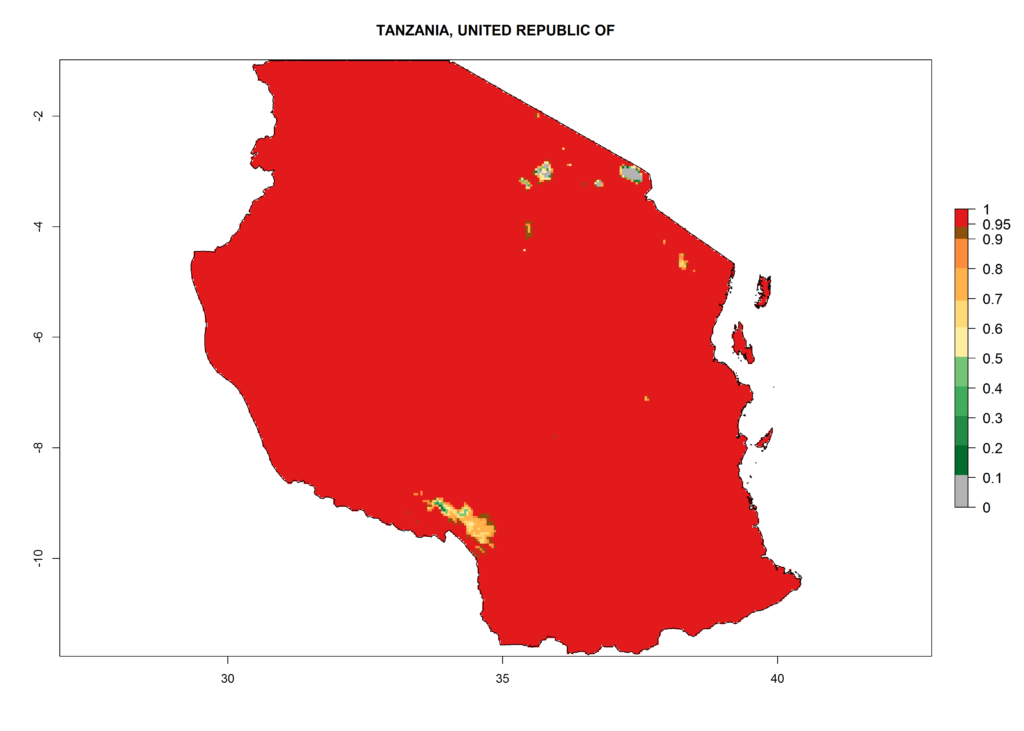
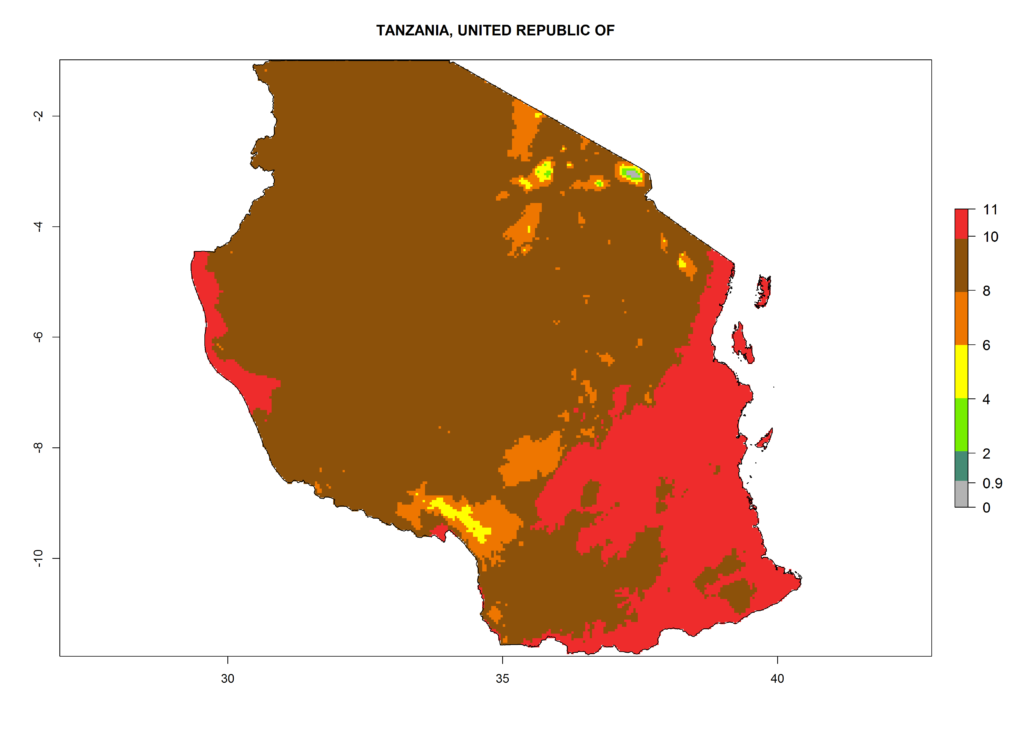
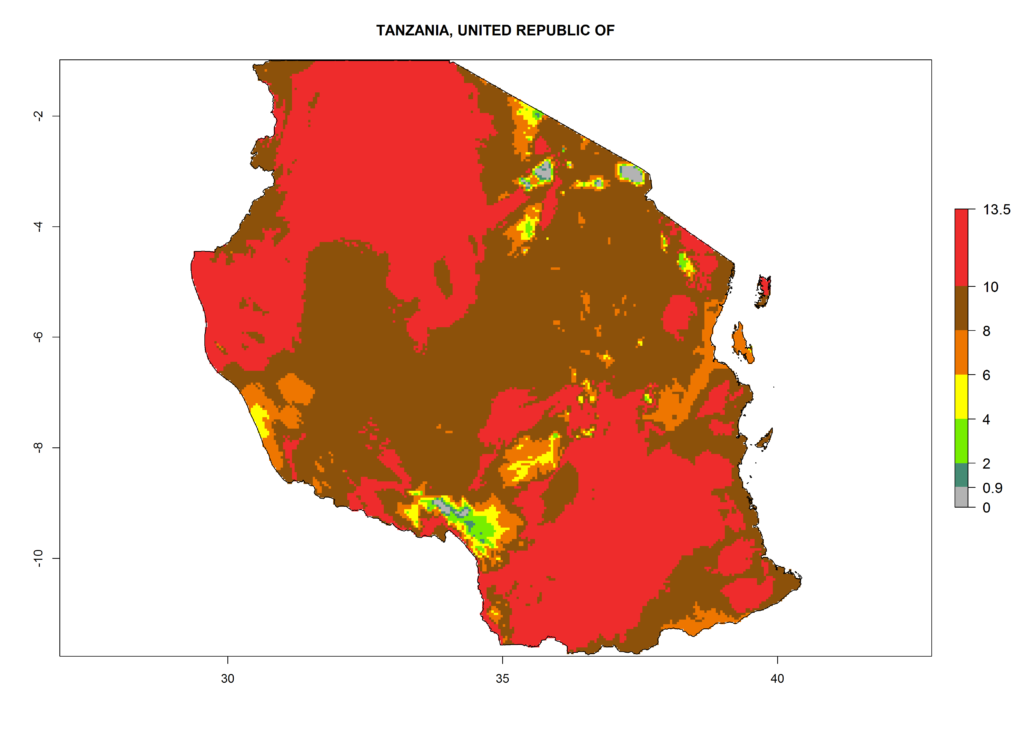
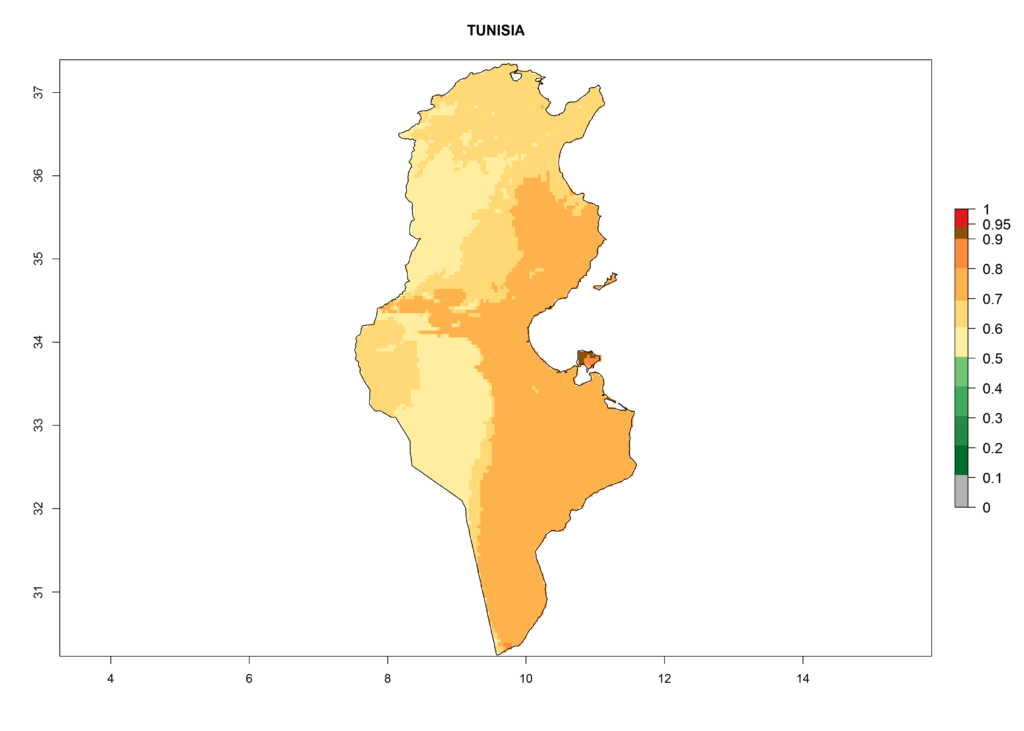
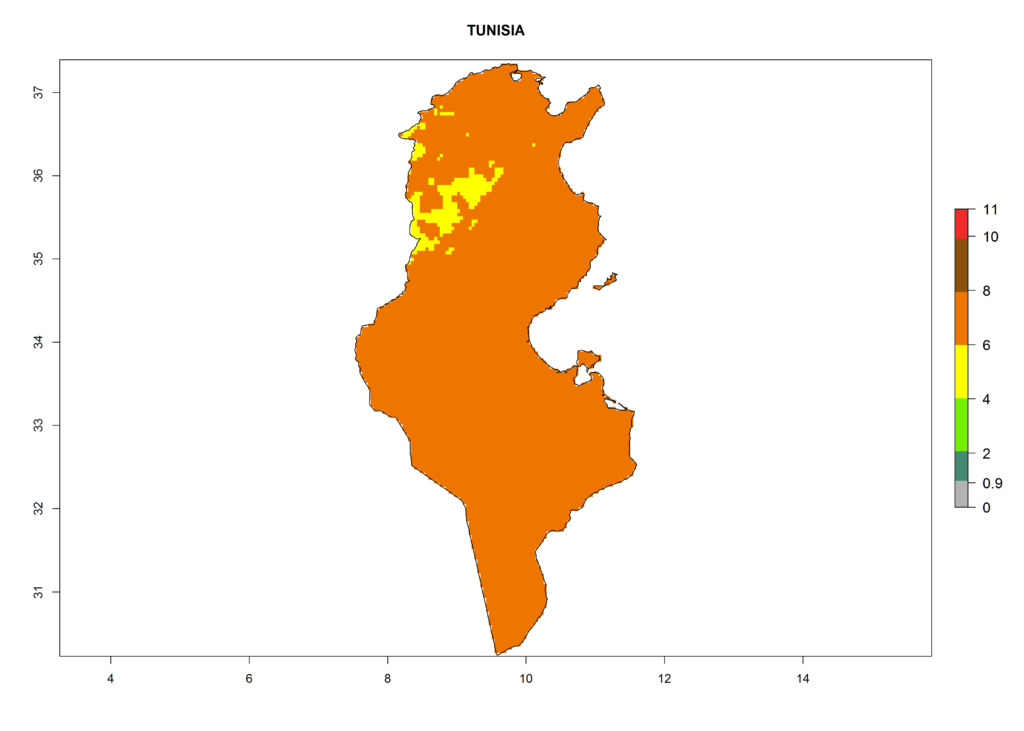
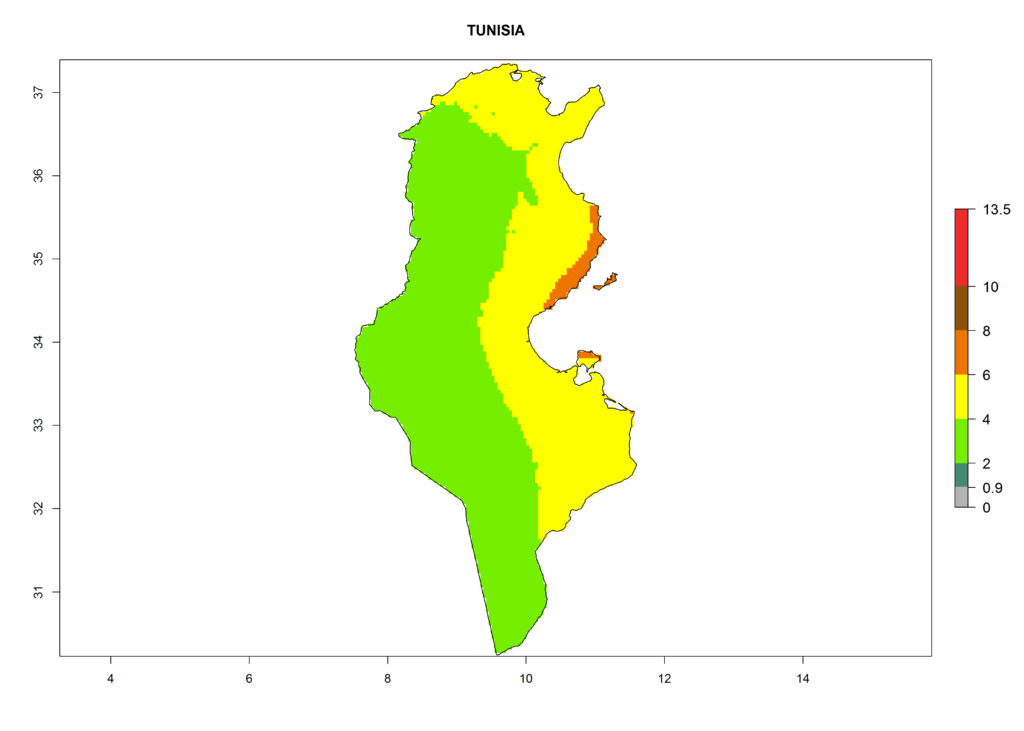
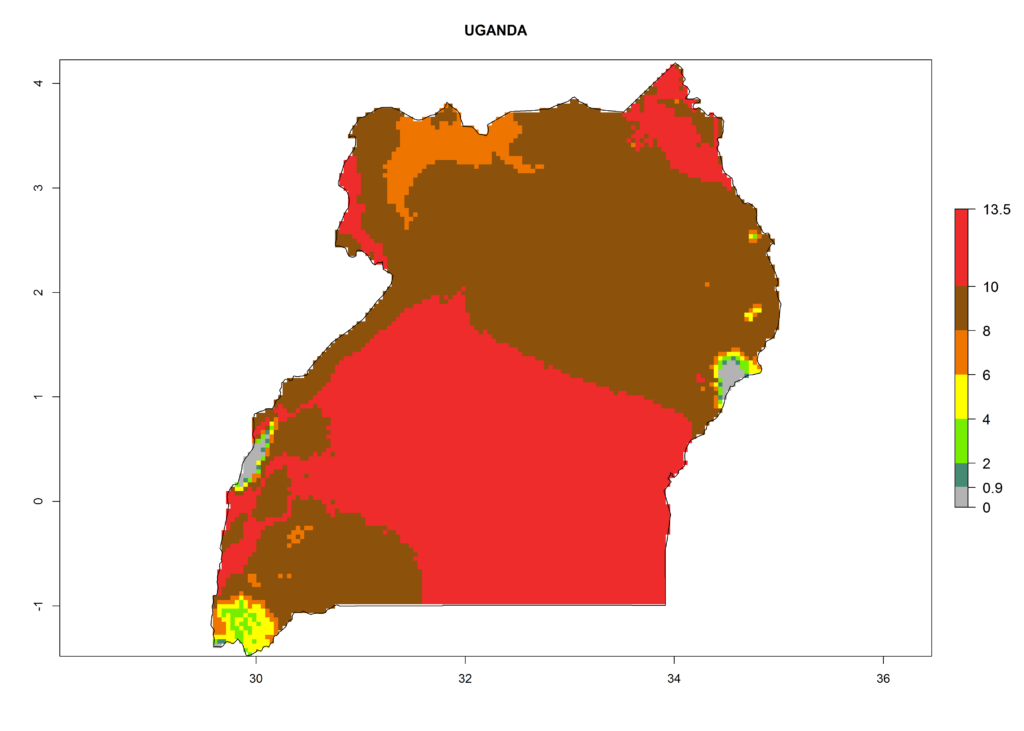
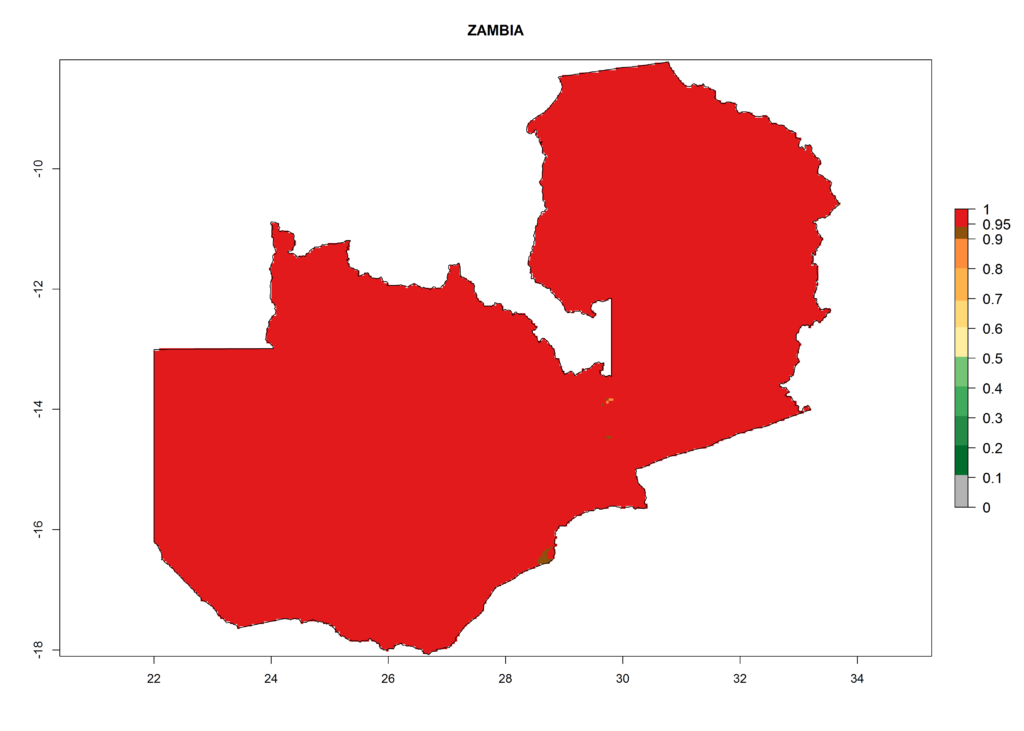
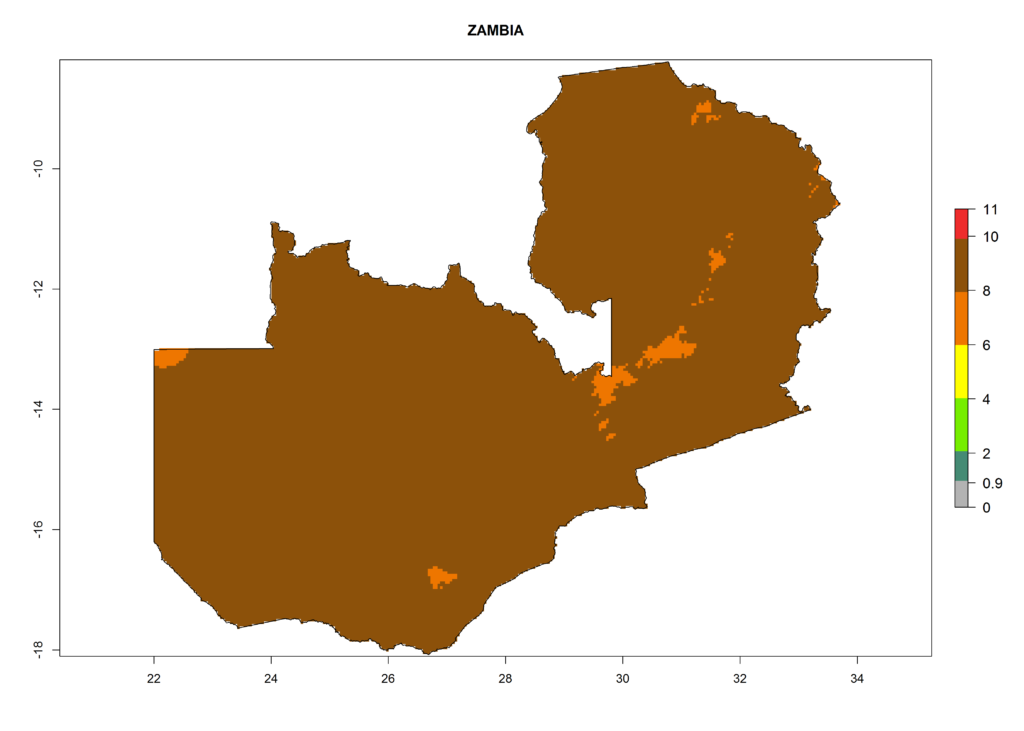
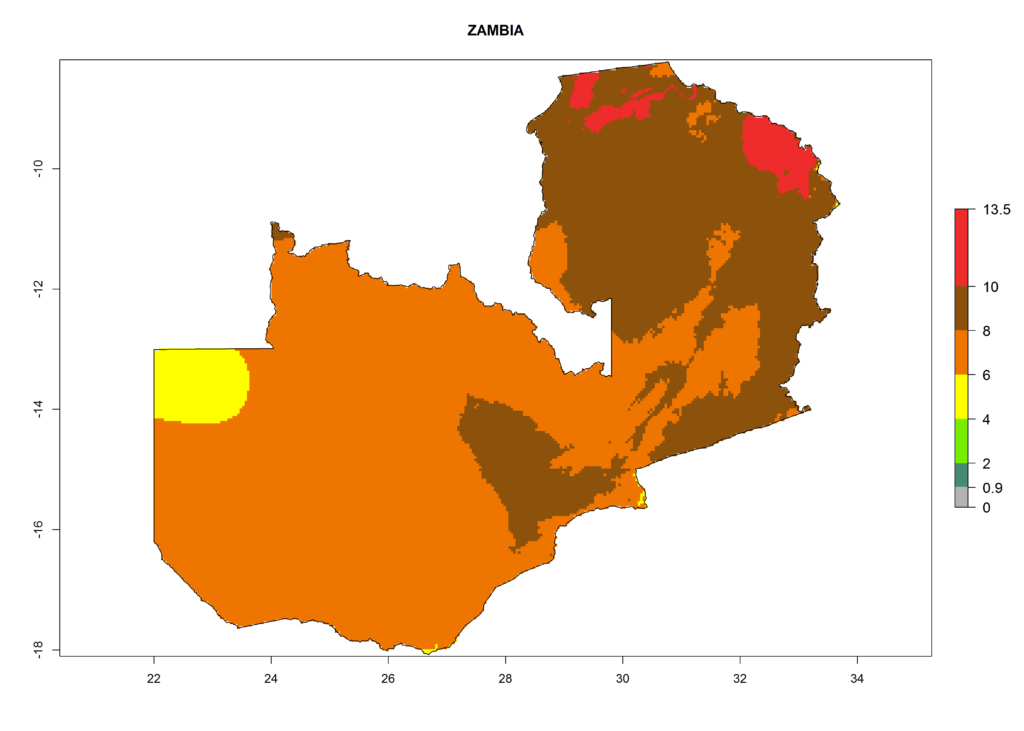
Under the current climate, T. vaporariorum represents a severe risk (ERI>0.95) in the countries of Central, Southern, East, and West Africa, which is associated with a GI>6–11 generations per year and an AI>6 (Fig. 5A, D). The highest risks of establishment (ERI>0.9–1, red zones) are observed in lowland production zones of each country (Fig. 6). Countries with a predicted high risk for establishment of T. vaporariorum under the present climate are Angola, Burundi, Congo Republic, DR Congo, Ethiopia, Kenya, Ghana, Madagascar, Malawi, Namibia, Rwanda, Tanzania, Uganda, Zambia, and Zimbabwe. Some areas where T. vaporariorum is established today show an ERI>0.95 (red) (Fig. 4), but it is also reported in countries with a lower likelihood of establishment and an ERI>0.6 (Algeria, Morocco, South Africa, Tunisia). Most of Tanzania is situated at an altitude of >1,600 masl and largely characterized by a temperate highland climate. Consequently, Tanzania presents the largest area at high risk of establishment, and T. vaporariorum is known to be damaging the country’s tomato production (Fig. 6). By contrast, some of the tropical countries (e.g., Algeria, Morocco, Mauritania) are at low risk of establishment (ERI<0.7). In southern zones in Tanzania, the number of generations is GI>8 per year and with AI>10 values (Fig. 6).
Figure 6. ERI, abundance (GI, potential damage), and activity (AI, potential population growth) of the greenhouse whitefly, Trialeurodes vaporariorum, in selected African countries according to model predictions of the year 2000. An ERI>0.6 is associated with potential permanent establishment.
Phytosanitary measures
In countries where T. vaporariorum is not already present, the enforcement of strict phytosanitary regulations may help to reduce the risk of this whitefly becoming introduced and established. Because of the difficulty of detecting low levels of infestation in consignments, it is best to ensure that the place of production is free of pests (OEPP/EPPO 1990). Particular attention is needed for consignments from countries where certain T. vaporariorum-listed viruses on the quarantine list are present.
Adaptation to risk avoidance at farm level
T. vaporariorum has high pathogenicity and virulence, causing great losses to crops. Such losses have led to the indiscriminate use of insecticides to try to control the pest, thereby also reducing its natural enemies. The direct impacts of whiteflies or through their transmission of viruses can be limited by considering the following control measures.
Monitoring pest populations. Plants should be visually inspected for signs of a whitefly infestation. A hand lens is useful for systematically inspecting a number of individual plants for the presence of eggs, nymphs, or adults. Both the upper and lower leaf surfaces should be inspected. Chemical control should be based on using action thresholds if three nymphs per leaf are observed. Trapping with yellow sticky cards is essential for a successful whitefly management program. The cards are used to detect and monitor population levels. As a general rule, one to four cards spaced evenly throughout 1,000 ft2 of greenhouse are sufficient. A generally acceptable threshold for whiteflies is one-half per card per day when the crop is young and two per card per day as the crop reaches maturity. Silver-painted pot spaces and silver polyethylene mulch have been successfully used to control whiteflies in greenhouses. The reflective materials were used in conjunction with yellow sticky cards or tape. The result was significantly enhanced trapping of whiteflies, relative to controls with sticky traps only.
Biological control. Several beneficials are used in the biological control of whiteflies. The parasitic wasp Encarsia formosa Gahan preys on immature whiteflies and is commonly used in greenhouses. E. formosa wasps kill whitefly nymphs in one of two ways: they either lay an egg inside the nymph, providing food for their offspring, or they kill the nymph right away and feed on the fluids inside of it. Delphastus pusillus LeConte is a very small, black ladybird beetle that attacks all stages of whiteflies but prefers eggs and nymphs. The females lay their eggs within clusters of whitefly eggs. Adults can consume 160 eggs or 12 large nymphs per day. A larva consumes 1,000 whitefly eggs during its development. These predators can be used in combination with Encarsia sp.
Biopesticides and biorational pesticides. Some microorganisms are used in whitefly control. For instance, the fungus Beauveria bassiana (trade names Naturalis-O and BotaniGard) is effective against eggs, nymphs, and adults. Thorough coverage of leaf undersides and correct timing of applications result in best control. Another fungus, Paecilomyces fumosoroseus (trade name PFR-97) is also commercially available. It controls whiteflies, aphids, and spider mites. Both B. bassiana and P. fumosoroseus need high relative humidity for best results. Several least-toxic botanicals and biorational pesticides have been evaluated for their effectiveness against different whitefly species. These include neem-based formulations (Neemazad and Azatin are two registered products), insecticidal soap (M-Pede), and horticultural oil. Enhanced whitefly control is achieved with thorough spray coverage. Wider plant spacing and removal of dead lower leaves improve pesticide coverage and pest control. Applications are carried out at early crop infestations to keep whitefly populations at low levels.
Insect growth regulators (IGRs) are other least toxic pesticide control options that typically kill insects by disrupting their development. They have a complex mode of action that precludes insects from rapidly developing resistance. IGRs usually take several days to have an effect on pest populations. Buprofezin has shown high efficacy (>90%) in controlling immature stages. Since IGRs do not affect adults, adult beneficials released into the greenhouse after an IGR application are not affected. IGRs are sometimes used together with biological control to keep pests below levels that are economically damaging.
Crop management. Crop rotation, growing only one crop per year, or removal of plant debris and weeds after harvest helps to reduce the numbers of whitefly nymphs and eggs. Maintaining a minimum distance between crops and use of certified planting material reduces the introduction and spread of TICV. The use of fine mesh protects infestation of whitefly in greenhouses.
Further reading
Basso, C., J. Franco, G. Grille, and C. Pascal. 2001. Spatial distribution of Trialeurodes vaporariorum (Homoptera: Aleyrodidae) on tomato plants. Plant Health Bulletin—Pest 27: 475–487. (In Spanish)
CABI. 2013. Trialeurodes vaporariorum data sheet. Crop protection compendium. Wallingford, UK: CAB International. Available from: http://www.cabi.org/isc/datasheet/54660.
Cardona, C., I. Rodriguez, J. Bueno, and X. Tapia. 2005. Biology and management of the whitefly Trialeurodes vaporariorum in field beans. International Center for Tropical Agriculture (CIAT). Department for International Development (DFID). Cali, Colombia. Publication No. 345, 50p. (In Spanish)
Duffus, J.E., H-Y. Liu, and G.C. Wisler. 1996. Tomato infectious chlorosis virus—a new clostero-like virus transmitted by Trialeurodes vaporariorum. European Journal of Plant Pathology 102: 219–226.
European Plant Protection Organisation (EPPO). 2014e. Trialeurodes vaporariorum. PQR database. Paris, France: European and Mediterranean Plant Protection Organization. Available from: http://www.eppo.int/DATABASES/pqr/pqr.htm
Gerling, D. 1990. Whiteflies: Their Bionomics, Pest Status and Management. Andover, UK: Intercept Press, 348 pp.
Gonzales, J.E., R. Moreno, M.D. Rodriguez, M.P. Rodriguez, E. Mirasol, J. Lastres, and C. Manzanares. 1996. Evolution of parasitism in Bemisia tabaci (Genn.) and Trialeurodes vaporariorum (West.) (Hemiptera: Aleyrodidae) in greenhouses of Almería. Boletin de Sanidad Vegetal Plagas 22: 373–389.
Johnson, M.W., L.C. Caprio, J.A. Coughlin, B.E. Tabashnik, J.A. Rosenheim, and S.C. Welter. 1992. Effect of Trialeurodes vaporariorum (Homoptera: Aleyrodidae) on yield of fresh market tomatoes. Journal of Economic Entomology 85(6): 2370–2376.
Jones, D. 2003. Plant viruses transmitted by whiteflies. European Journal of Plant Pathology 109: 195–219.
Kassis, G., and S. Michelakis. 1993. The effectiveness of biological control of the glasshouse whitefly Trialeurodes vaporariorum Westwood (Hom., Aleyrodidae) by Encarsia formosa Gahan (Hym., Aphelinidae). Journal of Applied Entomology 116(3): 298–302.
Loginova, E. 1990. Application of Applaud 25 WP against glasshouse whitefly (Trialeurodes vaporariorum). Selskostopanska Akademiya, Rasteniev”dni-Nauki (Bulgaria). Plant Science 27(3): 105–109.
Lenteren, J.C., and L.P.J.J. Noldus. 1990. Whitefly-plant relationships: Behavioral and ecological aspects. In: Whitefly: Their bionomics, pest status and management. D. Gerling, ed., 47–89. Andover, UK: Intercept Press.
McKee, G.J., F.G. Zalom, and R.E. Goodhue. 2007. Management and yield impact of the greenhouse whitefly (Trialeurodes vaporariorum) on California strawberries. HortScience 42(2): 280–284.
Mexia, A., E. Figueiredo, and M. do Céu Godinho. 2004. Natural control against pests on vegetables in Portugal: important species and their role. Pesticides and Beneficial Organisms. IOBC/wprs Bulletin 27(6): 1–8.
Morales, F.J., C. Cardona, J.M. Bueno, and I. Rodríguez. 2006. Integrated plant disease management caused by viruses transmitted by whiteflies. Cali, Colombia, International Center for Tropical Agriculture (CIAT), 43pp. (In Spanish)
Mound, L.A., and S.H. Halsey. 1978. Whitefly of the world: A systematic catalogue of the Aleyrodidae (Homoptera) with host plant and natural enemy data. Chichester, British Museum (Natural History): John Wiley & Sons, 340 pp.
Rodriguez, G.A., M. Hiller, and E. Williams. 1996. Action thresholds for the greenhouse whitefly, Trialeurodes vaporariorum (Westwood) (Homoptera: Aleyrodidae) in tomato. Revista Colombiana de Entomologia 22(2): 87–92.
Salazar, L.F., G. Müller, M. Querci, J.L. Zapata, and R.A. Owens. 2000. Potato yellow vein virus: its host range, distribution in South America and identification as a Crinivirus transmitted by Trialeurodes vaporariorum. Annals of Applied Biology 137: 7–19.
Zabudskaya, I.A. 1989. Biological control of the greenhouse whitefly, Trialeurodes vaporariorum (Homoptera, Aleyrodidae). Acta Entomologica Fennica 53: 73–76.