5.1.4 Biocontrol Agents Associated With Potato And Vegetable Pests![]() /Halticoptera arduine (Walker 1843)
/Halticoptera arduine (Walker 1843)
Synonyms: Dicyclus arduine (Walker 1843)
Halticoptera arduine (Walker 1843)
Taxonomic position: Hymenoptera, Pteromalidae: Miscogastrinae
Authors: N. Mujica, C. Prudencio, P. Carhuapoma, & J. Kroschel
Hosts
Halticoptera arduine is found parasitizing leafminer species (Diptera: Agromyzidae) of economic importance such as Amauromyza maculosa (Malloch) in Lacttuca sp. and Chrysantemum sp., Cerodontha dorsalis (Loew) in cereals; Japanagromyza Sasakawa in Phaseolus sp., Liriomyza huidobrensis (Blanchard), Liriomyza sativae Blanchard, and L. trifolii (Burgess) in vegetables; Liriomyza graminivora Hering in maize (Zea mays L.); and Liriomyza quadrata Malloch in potato (Solanum tuberosum L.) and tomato (Lycopersicum esculentum Mill.). Also, H. arduine has been recovered from leafminer flies of minor importance infesting wild plants such as Amauromyza Hendel sp., Calycomyza malvae (Burgess), Calycomyza Hendel, Chromatomyia platensis (Brethes), Liriomyza commelinae Frost, Liriomyza sabaziae Spencer, and Melanagromyza Hendel.
Morphology
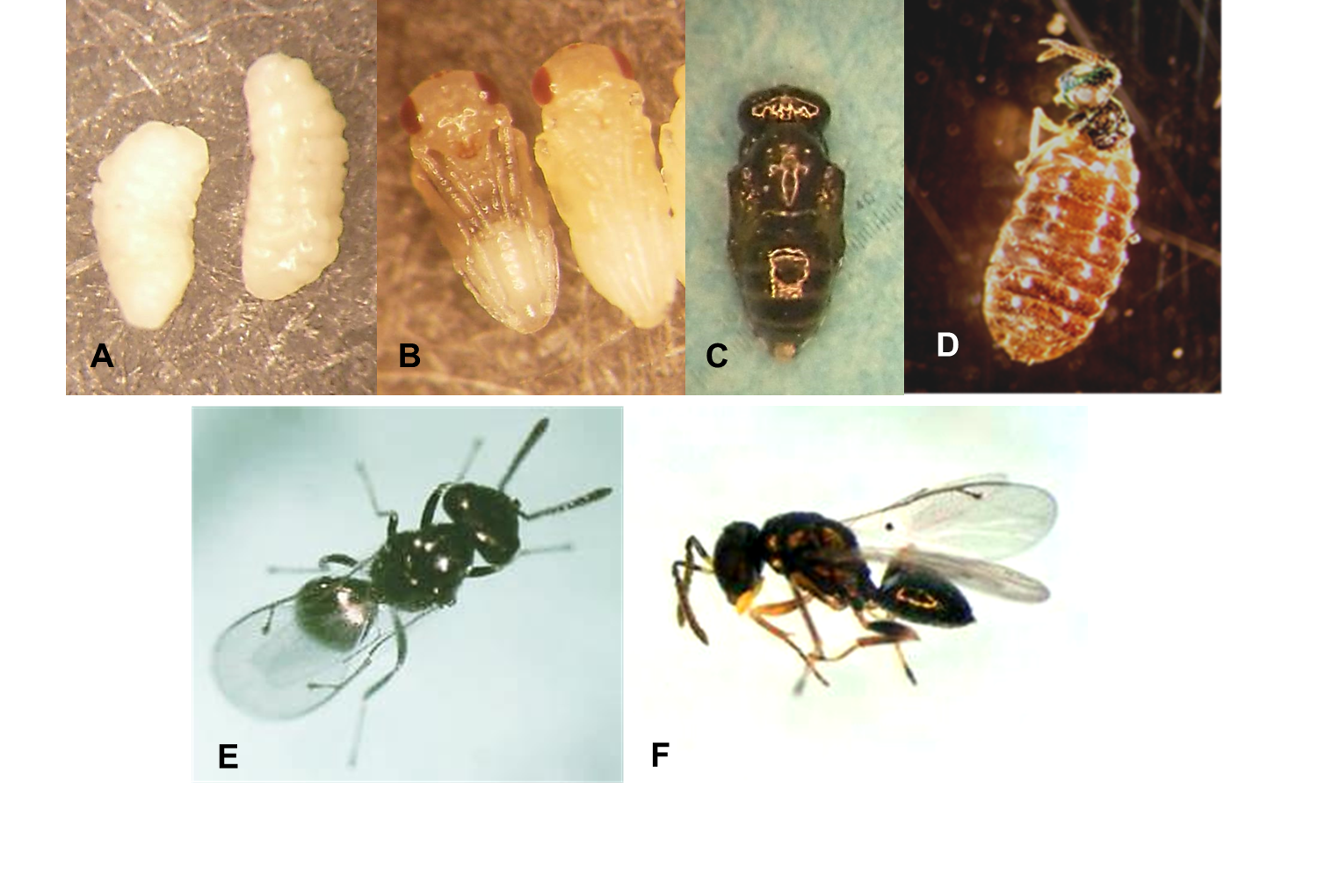
Egg
Eggs are 0.4 x 0.15 mm in size and are hymenopteriform; a caudal extension with three hooks (like an anchor) allows the egg to adhere to the internal organs of the host. Corium is transparent and smooth, pale yellow.
Larva
Four larval instars are described. First instar is hymenopteriform (0.35 x 0.15 mm) with 13 segments, including the conical cephalic capsule with triangular mandibles that are the most sclerotic part of the body. Second (1.93 x 1.04 mm) and third (2.29 x 1.30 mm) instars are vermiform (Photo 1A). Fourth instar is typically hymenopteriform with 13 segments, showing the oral region in the cephalic segment (2.49 x 1.34 mm).
Pupa
Pupation occurs within the host puparium. Pupa is hymenopteriform, sculpted, bright white, and 1.51 mm long (Photo 1B, C).
Adult
Tarsi are 5-segmented; antennae have 6 funicular segments and a complete notauli. Petiole is distinct though may be small. Median area of propodeum is virtually smooth. Parapsidal sutures are fully well marked in entirety. Pronotal collar is long, slightly margined. The species presents sexual dimorphism. Female: Body is completely dark with pale greenish, golden, and purple reflexes (Photo 1E) and dark scape antennae. Maxillary palpi and stipites are dark. Body is 1.32–1.68 mm long. Male: Body has purple and green reflections; stipites are quite apparent and yellow. Maxillary palpi are enlarged and yellow (Photo 1D, F), and scape has a yellow basal portion. Body is 1.32–1.58 mm long
Biology
Parasitism
H. arduine is a monoembrionic and koinobiont larval endoparasitoid of leafminer flies. (After oviposition the parasitoid offspring allows its host to continue developing, which is then killed when the parasitoid emerges as an adult.) Adults emerge in the early morning hours, breaking the puparium of the fly, usually between the 3rd and 4th segment (Photo 1D). They remain motionless for a short time until full sclerotization. Afterward, they become very active and females begin searching for hosts. Usually one egg is deposited—infrequently up to three eggs per host larva have been found, although only one offspring develops per host. Despite having a fixing anchor, eggs are free in the body cavity. Unfertilized females produce only males (arrenotokia parthenogenesis).
Temperature-dependent development
H. arduine completes its development from egg to adult at temperatures of 10°–30°C. Mean immature developmental period from egg to adult (using L. huidobrensis as host) decreased with increasing temperature, with 63 days at 10°C and 16 days at 30°C (see Annex 7.4.4). Mortality of egg-larvae was around 20–30% at temperatures of 15°–25°C. Pupae mortality was highest at extremes temperature, with 60% and 88% at 10°C and 30°C, respectively, compared with 14% at 20°C. Longevity of females and males decreased with increasing temperature, reaching a maximum of 47 and 35 days at 10°C and a minimum of 9 and 8 days at 30°C, respectively. Oviposition of H. arduine is significantly affected by temperature, showing a high variability in the progeny production. The lowest oviposition was observed at 10°C, with an average of 28.2 offspring/female and the highest at 25°C, with average of 75.6 offspring/female. The sex ratio was highly affected by temperature, with a predominance of males at 10°, 15°, 25°, and 30°C. Only at 20°C was a nearly balanced female:male sex ratio of 0.96:1 observed.
The functions that had been established to describe the development time and rate, mortality, and reproduction were compiled into an overall phenology model, and the following life-table parameters were calculated (see Annex 7.4.4). The intrinsic rate of increase (r) had positive values between 9.5°C (r=0.005) and 30°C (r= 0.004). The finite rate of increase peaked at 25°C (λ=1.08) and was smallest at 9.5°C and 30°C (λ=1.004), indicating that population decreases below and above these temperatures. Highest values for the gross reproduction rate and net reproductive rate (Ro) were found at 20°C and 21°C, with 14.6 and 41.8 female offspring per female, respectively. The mean generation time (T) decreased with temperature and was shortest at 29°C, with 9 days from egg to egg. The shortest mean development time (Dt) was observed at 29.5°C (21 days). The optimum temperature for overall population growth ranged 23°–27oC. H. arduine presents a higher intrinsic and finite rate of increase than L. huidobrensis (see section 4.3.1), confirming its potential as a biological control agent on this pest.
Economic impact in pest control
Along the Peruvian coast, H. arduine has been found to parasitize economic leafminer species with different parasitism rates: Amauromyza maculosa (15.4%), Cerodontha dorsalis (66.7%), Japanagromyza sp. (30.1%), L. huidobrensis (49.3%), L. sativae (51.0%), L. graminivora (23%), and Melanagromyza sp. (33.3%). All these leafminer species have been found on a total of 25 crops. In Jujuy, Argentina, H. arduine was found parasitizing L. huidobrensis on 22 vegetable crops at altitudes of 2,000–2,950 masl. In lowland conditions and under high L. huidobrensis infestation (cold months from May to September), parasitism rate of H. arduine ranged 17–28%. In highland conditions (3,200 masl), H. arduine presents high parasitism rates (24–52%) year round. In classical biological control programs, H. arduine was imported from Peru and introduced to Kenya and released as an exotic parasitoid. This was done in combination with the eulophid Chrysocharis flacilla Walker and the braconid Phaedrotoma scabriventris Nixon (see sections 5.1.6 and 5.1.5) to control the invasive leafminer flies Liriomyza huidobrensis, L. sativae, and L. trifolii (see sections 4.3.1–4.3.3). H. arduine was released in vegetable production sites at different altitudes in 2012 and 2013. Monitoring and recovery surveys in 2013 and 2014 indicated that the parasitoid was established in all release sites and had spread on a 10-km radius 1 year after release. Specific parasitism of the introduced parasitoid is still low; an increase is expected after its acclimatization to its new environments.
Geographical distribution
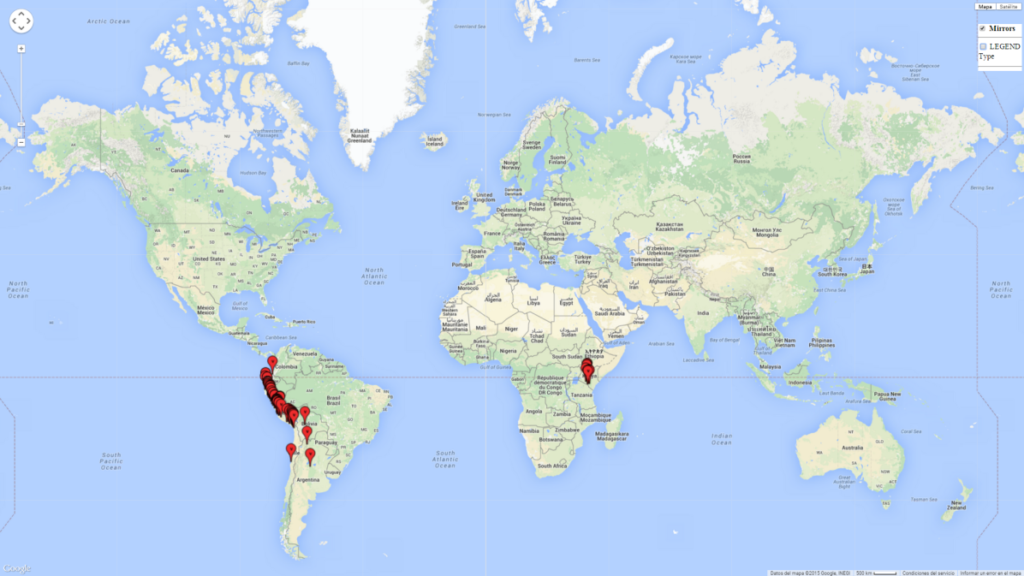
Possible regions of origin: Neotropics: Argentina, Chile, Peru, Bolivia, and Ecuador. Also reported from Central America in Costa Rica.
Introduced and established: In Kenya for the classical biological control of L. huidobrensis, L. sativae, and L. trifolii (Fig. 1)
H. arduine is adapted to wide ecological amplitude. The species occurs in coastal regions of Peru and Chile from sea level to 500 masl, as well as in Andean highlands at 2,950 masl in Argentina or 4,042 masl in Peru. The region of Jujuy (Argentina), where H. arduine occurs at 2,000–2,950 masl, has a warm climate with average temperatures of 18°–20°C (max. 30°C, min. 4°C). In Peru, H. arduine is found parasitizing L. huidobrensis at 3,250–4,042 masl, with mean temperatures of 8°C in winter and 24°C in summer. The Peruvian coast is characterized by a semi-warm, subtropical desert climate with an average temperature of 19°C in the central and southern regions, and a very warm tropical desert with temperatures averaging 24°C in the northern region, where H. arduine is recovered from L. huidobrensis and L. sativae, respectively.
Potential establishment and efficiency under current and future climates
Changes in global establishment and future distribution
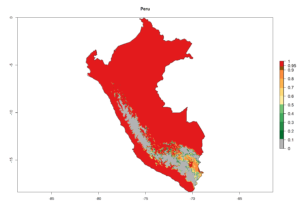
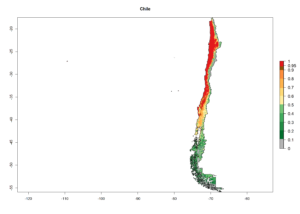
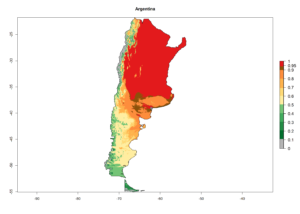
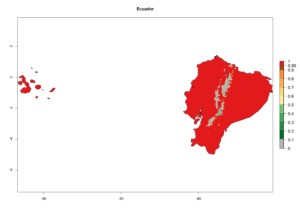
An establishment index (EI)=1 indicates survival of the parasitoid throughout the year (i.e., the likelihood of long-term establishment for classical biological control is very high in these regions). A high EI (>0.95) of H. arduine is simulated for regions of the parasitoid origin, along the Peruvian coast, the northern part of Chile (Arica, Coquimbo), and Ecuador (Carchi). Also, areas where H. arduine has been introduced in Kenya are well reflected by an EI>0.95 (Fig. 2). In zones where the EI drops below the maximum number of 1, the likelihood of long-term establishment is reduced. However, H. arduine is already distributed under open-field conditions in high elevation regions of South America, with an EI>0.6–0.8 (light and dark orange zones) as in Prepuna of Argentina, Andean valleys in Peru (Junín), and Bolivia (Cochabamba).
| Peru | Chile | Argentina |
| Ecuador | Bolivia | Kenya |
Figure 2. EI of Halticoptera arduine in countries where establishment has been reported, according to model predictions for the year 2000. An EI>0.6 indicates regions with permanent establishment.
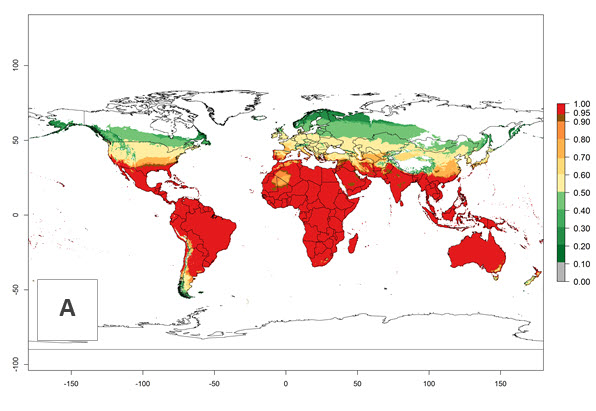
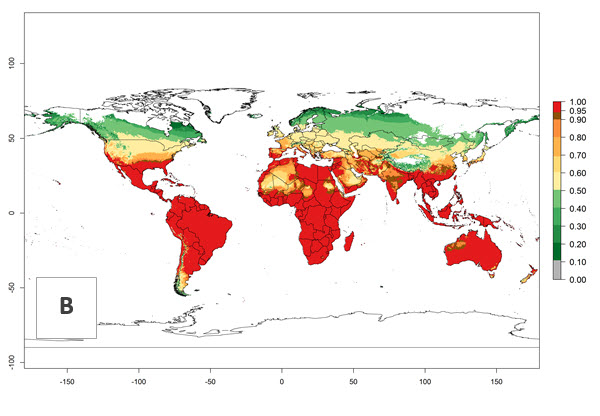
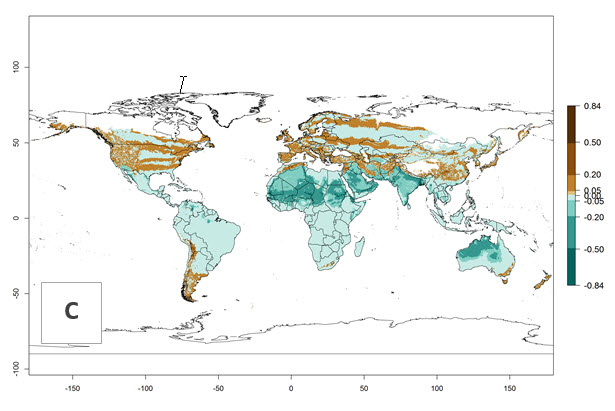
Global predictions for the year 2000 indicate a high potential of establishment of H. arduine (EI>0.95) in most tropical and subtropical zones of Africa, America, Asia, and Australia (Fig. 3A), where the leafminer fly species L. huidobrensis (section 4.3.1), L. sativae (section 4.3.2), and L. trifolii (section 4.3.3) have a high ERI and a medium establishment potential (EI>0.7) for European Mediterranean countries and central China. Global predictions for the year 2050 indicate a slight decrease in the establishment potential (<-0.05) of H. arduine in most of the tropical and subtropical zones. The parasitoid will, however, continue to have a high potential of establishment (EI>0.95) and for successful naturalization in biocontrol programs of Liriomyza sp. in many countries and vegetable-growing regions (Fig. 3B, C). Further, its range of establishment and use is expected to expand to higher altitudes (EI>0.8), as in the Andes of Peru, Bolivia, Chile, and Argentina. Similar establishment is predicted for the Mediterranean region, central China, and southern parts of North America (USA) and South America (Chile, Argentina). Although conditions for establishment might improve in more temperate regions of Asia, Europe, and North America, the EI<0.6 is still very low, suggesting that the parasitoid could be only successfully used for inundative releases in protected crops (Fig. 3B, C).
 |
 |
 |
|
Figure 3. Changes in establishment and potential distribution worldwide of Halticoptera arduine, according to model predictions, using the EI for the years 2000 (A), and 2050 (B), and changes of the EI between 2000 and 2050 (C). An EI>0.6 indicates regions with permanent establishment.
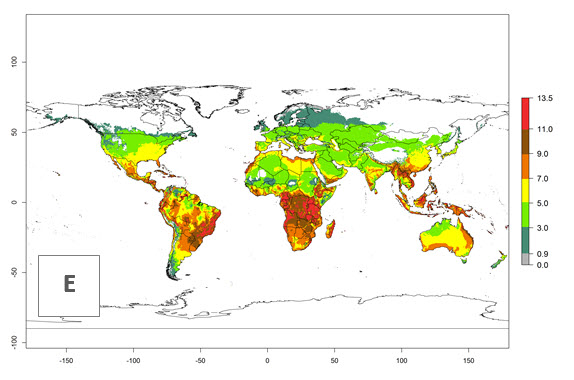
Changes in global abundance
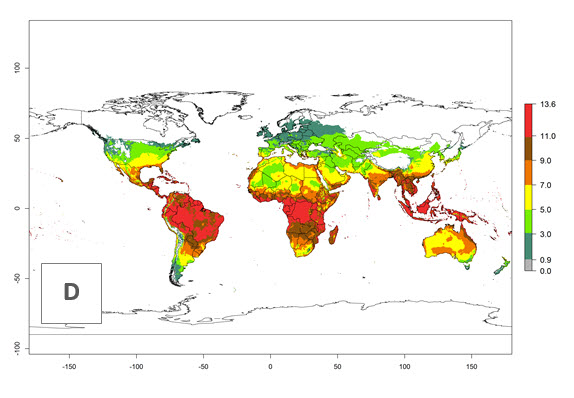
The generation index (GI) for the year 2000 estimates 9–15 generations per year for most tropical regions and 5–11 generations per year for most subtropical regions of America, Africa, Asia, and Australia (Fig. 4A). For temperate regions, 2–5 generations per year are predicted to develop. The observed number of generations in countries where H. arduine is naturally distributed ranges 2–5 per year in the highlands of Ecuador, Peru, Bolivia, and the prepuna of Argentina; 5–7 per year at the northern coast of Chile; 7–9 per year in the northern province of Cordoba-Argentina and central and southern coast of Peru; and 9–11 per year at the northern coast of Peru.
The GI change indicates that for most of the tropical zones of the world, the abundance of H. arduine will decrease slightly by 1 generation per year, and in some areas (especially tropical zones in South America) by 1–2 generations per year. But the parasitoid will still maintain a high abundance by 2050 (Fig. 4B, C). In contrast, for most subtropical regions, an increase of 1 generation per year is predicted. In the highlands of the Andean region, southwest of Brazil, Mediterranean coast, and sub-Saharan Africa (SSA), an increase of 1–4 generations per year is predicted.
The activity index (AI) highlights not only the establishment but also the potential spread of an insect and, in the case of parasitoids, its potential to efficiently control its host. Global maps of the AI in year 2000 estimates the highest activity of H. arduine in tropical regions (AI>9–13.6), followed by subtropical regions (AI>5–9) (Fig. 4D). The potential population growth in countries where H. arduine is naturally distributed today ranges AI>3–7 in Andean highlands to AI>7–11 at the Peruvian coast. Predictions of changes for the year 2050 temperature scenario show a strong reduction in the potential growth of H. arduine in most tropical regions up to -6, with the highest reduction in some areas of South America (up to -9.8) (Fig. 4E, F). However, in the region of origin, an increase by a factor of 2–4 is expected in highland Andes and coastal areas of Chile and Peru. A similar increase is also predicted for regions in East and Southern Africa.
| GI | AI | |
| 2000 | 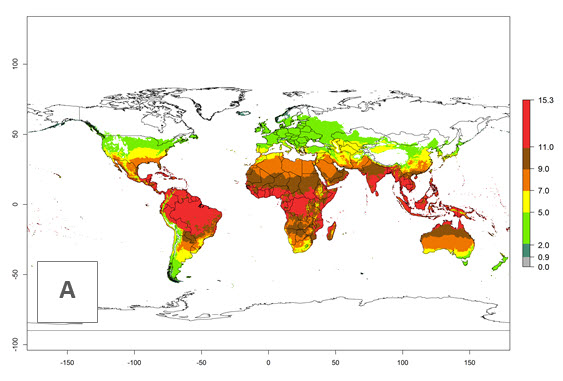 |
 |
| 2050 | 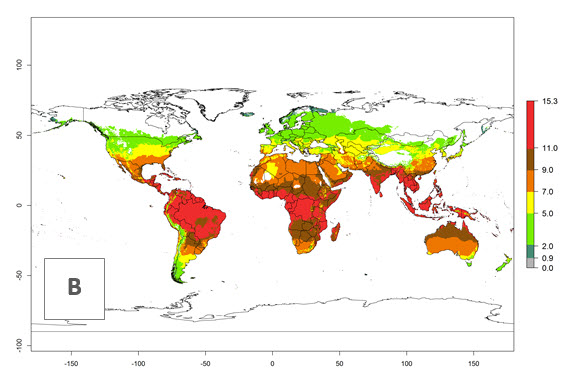 |
 |
| Index change (2000 – 2050) | 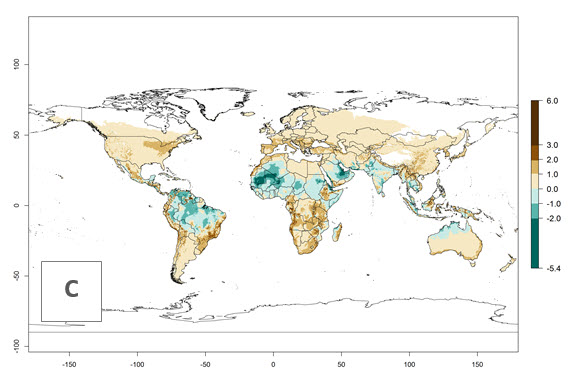 |
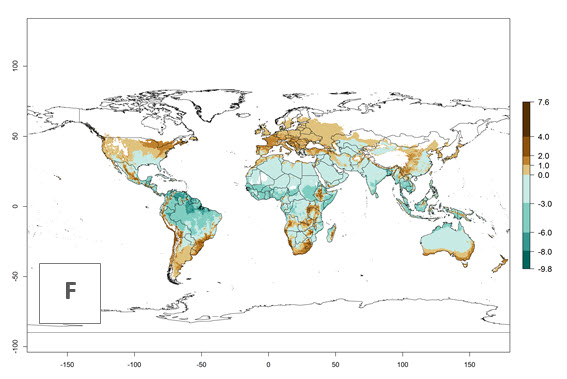 |
Figure 4. Changes in abundance (GI, number of generations/year) and activity (AI, potential population growth) of Halticoptera arduine worldwide according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
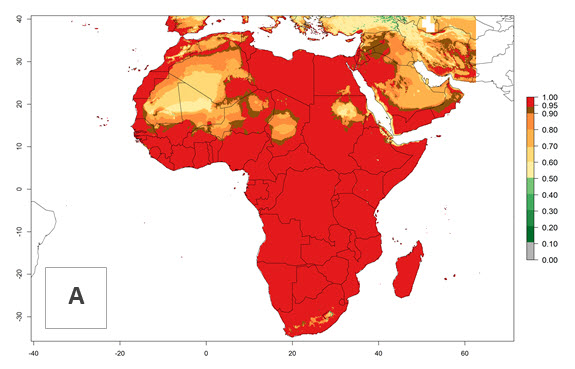
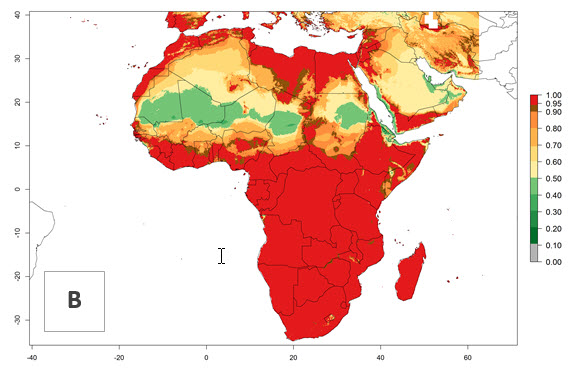
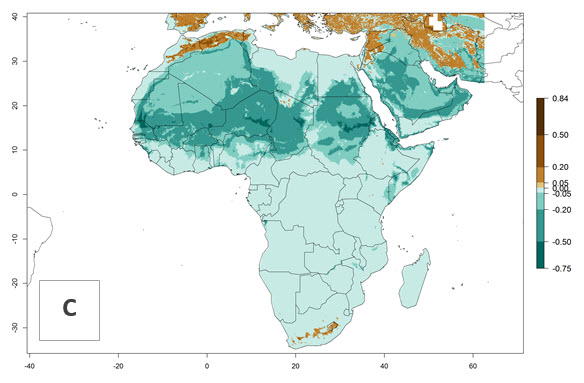
Changes in regional establishment and distribution in Africa
In Africa, H. arduine was introduced to Kenya and its establishment is confirmed. A successful establishment (EI>0.95) could be expected in almost all African countries (Fig. 5A). During climate change, H. arduine will maintain a high establishment potential (EI>0.95), although for the year 2050 a slight decrease in the establishment potential (<-0.05) is predicted for most regions in East, Southern, and Central Africa (Fig. 5B, C). The Sahara region will present unfavorable temperature conditions for development of H. arduine, as a considerable reduction in its establishment potential (up to -0.5) is expected. Likewise, Liriomyza species will also have less potential to establish, and its pest status will decrease in this region (see section 4.3.1).
 |
 |
 |
|
Figure 5. Change in establishment and potential distribution of Halticoptera arduine in Africa according to model predictions, using the EI for the years 2000 (A) and 2050 (B) and changes of the EI between 2000 and 2050 (C). An EI>0.6 indicates regions with permanent establishment.
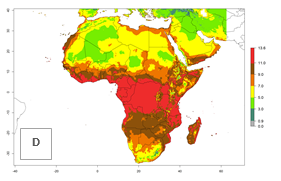
Changes in regional abundance in Africa
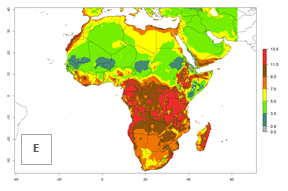
The GI for the year 2000 estimates 11–15 generations per year for tropical regions of Africa and 7–11 generations per year for most subtropical regions (Fig. 6A). For the Mediterranean region of Morocco, Algeria, and Tunisia, as well as for temperate climates of South Africa, 5–7 generations per year are predicted. The lowest abundance (GI>2–7 generations per year) is expected for highlands of East Africa (Ethiopia, Kenya, Tanzania). Predictions for the year 2050 scenario indicate an increase of 1–3 generations per year southward (from equatorial Africa to South Africa) and northward (northern African countries) (Fig. 6B, C). The GI is strongly correlated with the AI. The activity is expected to increase (up to 5) in some areas of Africa; especially in Ethiopia, Uganda, Kenya, Rwanda, Burundi, Tanzania, Malawi, Zambia, South Africa, Namibia, and Angola, where a high H. arduine activity of AI>9 is projected (Fig. 6E, F).
| GI | AI | |
| 2000 | 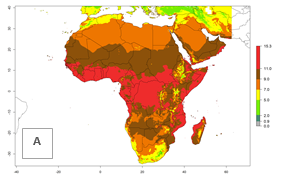 |
 |
| 2050 | 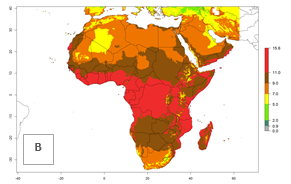 |
 |
| Index change (2000 – 2050) | 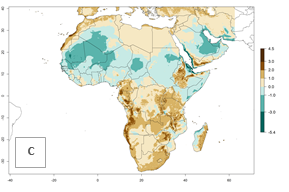 |
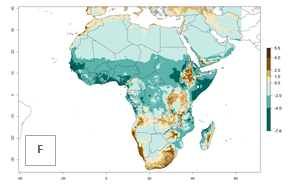 |
Figure 6. Changes in abundance (GI, number of generations/year) and activity (AI, potential population growth) of Halticoptera arduine in Africa according to model predictions, using the GI (A, B) and the AI (D, E) for the years 2000 and 2050, and the absolute index change (C, F).
Potential release areas in Africa
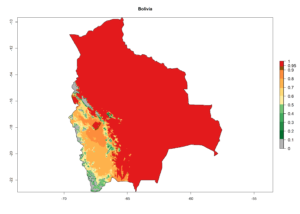
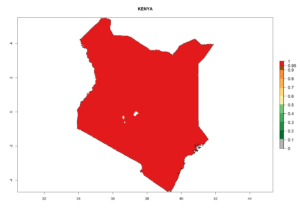
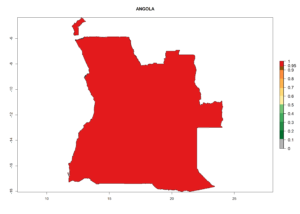
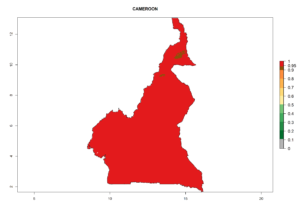
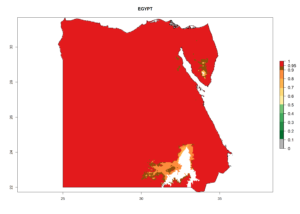
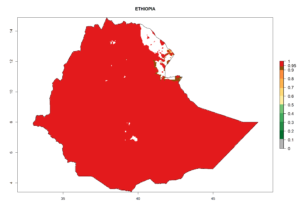
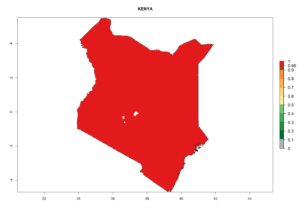
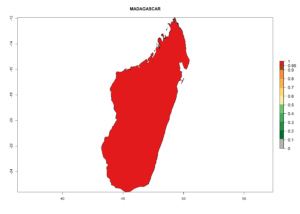
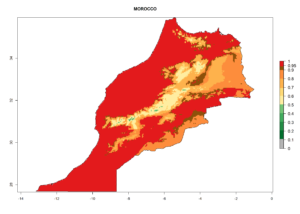
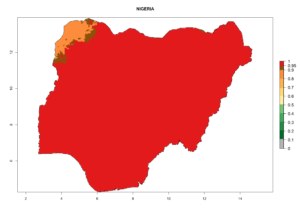
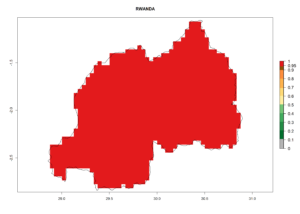
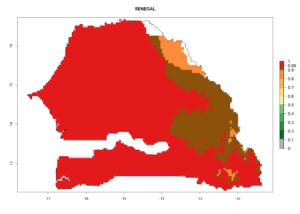
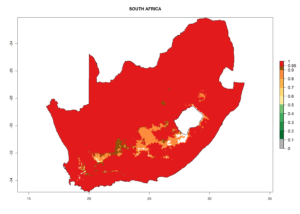
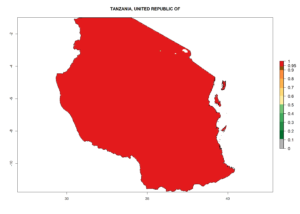
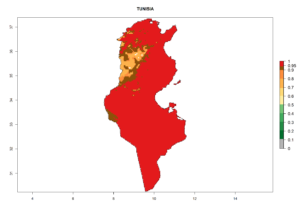
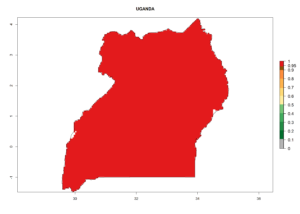
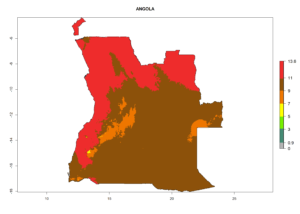
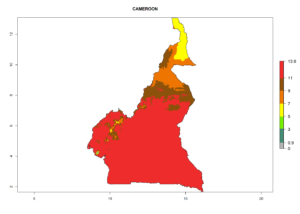
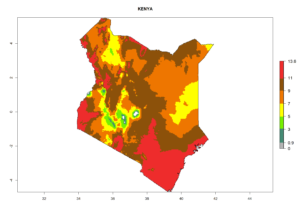
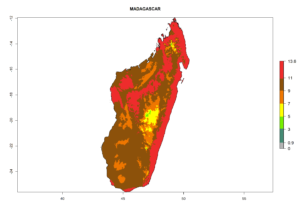
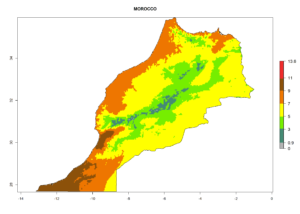
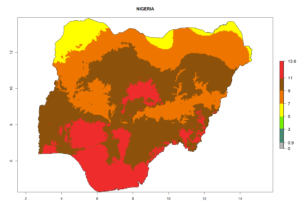
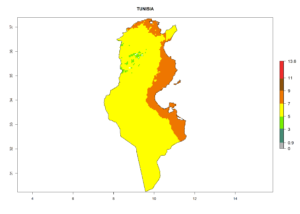
Considering the highest EI, GI, and AI of L. huidobrensis, L. sativae, and L. trifolii in areas of Africa (see section 4.3.1), potential countries to release H. arduine under the current climate are mainly those of East (Ethiopia, Kenya, Uganda, Rwanda, Tanzania, Madagascar); Central (Angola); and West (Senegal, Nigeria, Cameroon) Africa. In these countries, the likelihood of establishment of H. arduine is expected to be very high (EI>0.95) and associated with a GI>9 (i.e., more than 9 generations per year) and an AI>9 (Fig. 7). H. arduine could be also considered for releases in the Mediterranean region (Morocco, Tunisia, Egypt) and in Southern Africa, due to its high establishment potential (EI>0.95) but with a lower GI (5–9 generation per year) and AI (3–9).
| EI | GI | AI |
| a) Angola | 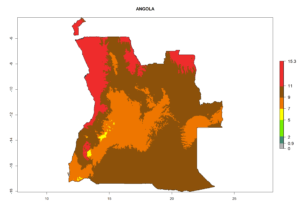 |
 |
| b) Cameroon | 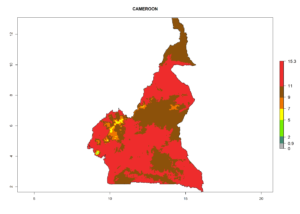 |
 |
| c) Egypt | 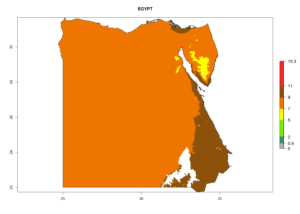 |
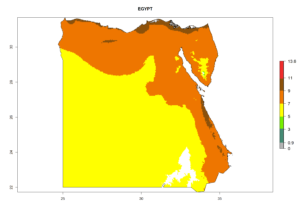 |
| d) Ethiopia | 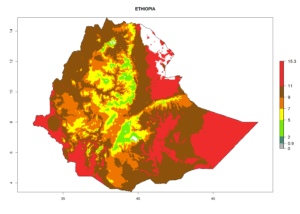 |
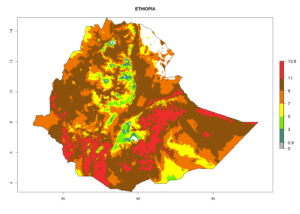 |
| e) Kenya | 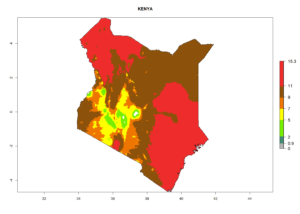 |
 |
| f) Madagascar | 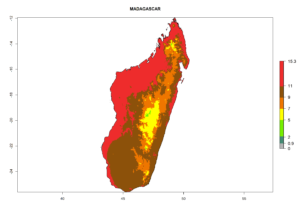 |
 |
| g) Morocco | 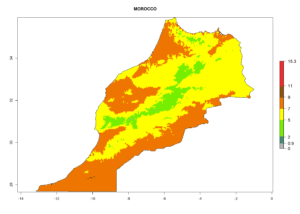 |
 |
| h) Nigeria | 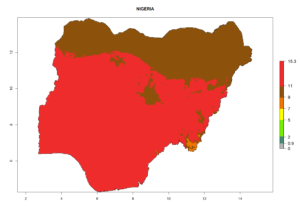 |
 |
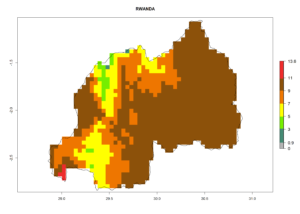
| i) Rwanda | 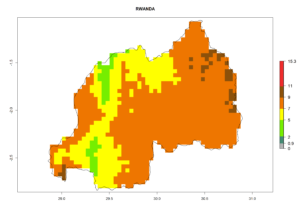 |
 |
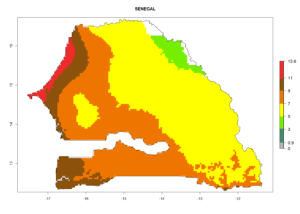
| j) Senegal | 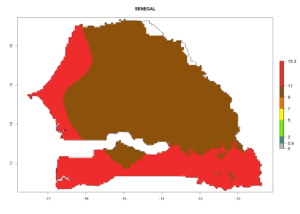 |
 |
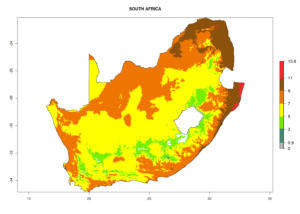
| k) South Africa | 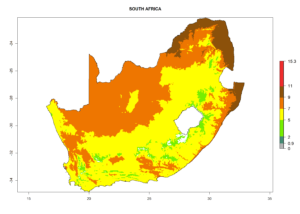 |
 |
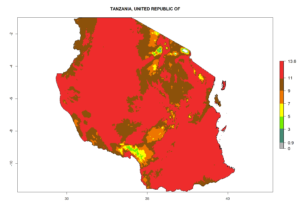
| l) Tanzania | 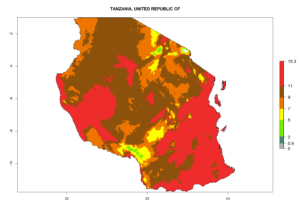 |
 |
| m) Tunisia | 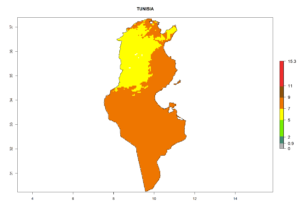 |
 |
| n) Uganda | 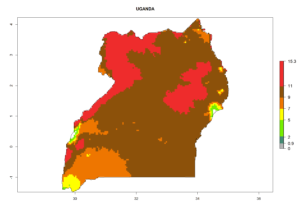 |
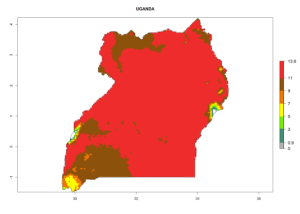 |
Figure 7. EI, abundance (GI, number of generations/year), and activity (AI, potential population growth) of Halticoptera arduine in selected African countries according to model predictions for the year 2000. An EI>0.6 indicates regions with permanent establishment.
Risks to non-targets
No risks are reported. H. arduine is a parasitoid of Agromyzidae leafminer flies with a wide host range. This also includes species that are non-agricultural pests. In the region of evolution the parasitoid lives in a natural balance and equilibrium with any kind of leafminer host, where (to our knowledge) it has not caused the extinction of any species. After its release in new agro-ecosystems, it is hoped that the species will adapt and naturalize in its new environment in order to achieve high impact by reducing the infestation of agricultural Agromyzidae pests such as L. huidobrensis, L. sativae, and L. trifolii. In addition to parasitism of target pests, the introduced parasitoid may also parasitize local leafminer species occurring in the natural environment. This, however, is unlikely to cause an extinction of a species.
Further reading
Aguilera, A. 1972. Biología de Liriomyza langei Frick (Dip.: Agromyzidae) y evaluación de los parásitos que emergen del puparium. Idesia 2: 71–85.
Arellano, G., and I. Redofi de Huiza. 1989. Biology of Halticoptera arduine (Hym. Pteromalidae), parasitoid of Liriomyza huidobrensis (Diptera, Agromyzidae). Revista Peruana de Entomología 31: 95–101.
Costa-Lima, T.C. 2011. Bioecologia y competencia de dos especies de parasitoides neotropicales (Hymenoptera: Braconidae y Eulophidae) de Liriomyza sativae Blanchard, 1938 (Diptera: Agromyzidae). PhD diss., Universidad de Sao Paulo, Sao Paulo, Brazil.
De Santis, L. 1985. Las especies peruanas de Halticoptera (Insecta, Hymenoptera, Pteromalidae). Revista Peruana de Entomología 28: 1–3.
Mujica, N. 2007. Malezas hospederas de moscas minadoras (Diptera: Agromyzidae) y sus parasitoides en el ecosistema de papa en La Molina. MSc thesis, Universidad Nacional Agraria La Molina, Lima, Peru.
Mujica, N., and J. Kroschel. 2011. Leafminer fly (Diptera: Agromyzidae) occurrence, distribution, and parasitoid associations in field and vegetable crops along the Peruvian coast. Environmental Entomology 40(2): 217–230.
Mujica, N., C. Prudencio, C. Valencia, L. Rodriguez, K. Fiaboe, and J. Kroschel. 2013. Potential of hymenopteran parasitoids for classical biocontrol of leafminer flies (Diptera: Agromyzidae). In: 4th International Symposium on Biological Control of Arthropods, March 4–8, Pucon, Chile.
Neder de Roman, E. 2000. The relationship of Halticoptera arduine (Walker) (Hym. Pteromalidae)-Liriomyza huidobrensis Blanchard (Dipt. Agromyzidae) in the highlands of Argentina. Idesia 18: 49–60.
Prudencio, C. 2012. Efecto de la temperatura en la biología de Halticoptera arduine (Walker) (Hymenóptera: Pteromalidae) parasitoide de Liriomyza huidobrensis (Blanchard) (Diptera: Agromyzidae) y estimación de parámetros de crianza. BSc thesis, Universidad Nacional Federico Villareal, Lima, Peru.
Sanchez, G., and I. Redolfi de Huiza. 1989. Liriomyza huidobrensis and its parasitoids in potato cultivated in Rimac and Canete Valleys, Peru, 1986. Revista Peruana de Entomología 31: 110–112.